Physical Properties of Alkenes
Alkenes are a group of hydrocarbons in which one of the carbon to carbon bonds in the chain is a double bond. The double bond causes the carbon chain to hold a lesser number of hydrogen atoms than possible, thus they are called unsaturated. The degree of unsaturation is dependent on the number of double bonds that exist in the molecule.
Sometimes referred to as olefins, alkenes have the general formula of CnH2n. For example, a five carbon alkene would have the formula C5H10. The name of the alkene would be dependent on the placement of the double bond in the carbon chain but would have the ~ene suffix to denote the existence of the pi bond.
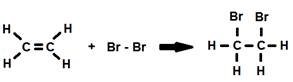
Alkenes are generally more reactive than alkanes due to the high reactivity of the double bond. Addition reactions are very common for alkenes where the double bond is broken and the open positions on the carbon atoms are filled by two other elements.
Alkenes are nonpolar, like alkanes, and therefore have London dispersion forces as a main means of attraction. This causes the melting and boiling points of alkenes to be very similar to their alkane counterpart. The larger the alkene, the more dispersion forces are present, and the higher the melting and boiling point. Alkenes are insoluble in water due to their nonpolar nature but will dissolve in organic solvents, such as carbon tetrachloride.
Sometimes referred to as olefins, alkenes have the general formula of CnH2n. For example, a five carbon alkene would have the formula C5H10. The name of the alkene would be dependent on the placement of the double bond in the carbon chain but would have the ~ene suffix to denote the existence of the pi bond.
Alkenes are generally more reactive than alkanes due to the high reactivity of the double bond. Addition reactions are very common for alkenes where the double bond is broken and the open positions on the carbon atoms are filled by two other elements.

Alkenes are nonpolar, like alkanes, and therefore have London dispersion forces as a main means of attraction. This causes the melting and boiling points of alkenes to be very similar to their alkane counterpart. The larger the alkene, the more dispersion forces are present, and the higher the melting and boiling point. Alkenes are insoluble in water due to their nonpolar nature but will dissolve in organic solvents, such as carbon tetrachloride.
|
Related Links: Chemistry Organic Chemistry Spectroscopy Alkane Structure and Bonding |
To link to this Physical Properties of Alkenes page, copy the following code to your site:
