1-Hexanol Formula
1-Hexanol is an organic alcohol formed by a 6 carbon chain and used in the perfume industry.
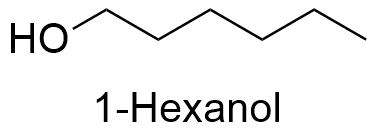
Formula and structure: The chemical formula of 1-hexanol is C6H14O. It is formed by a six-carbon linear chain with the formula CH3(CH2)5OH.The molecular mass is 102.18 g mol-1. 1-Hexanol is one of the several isomers of 6-carbon-alcohols, for example: 2-hexanol, 3-hexanol, etc. The chemical structures can be written as below, in the common representations used for organic molecules.
Occurrence: 1-Hexanol has been found in some pheromones of insects as bees and it is suspected to be a component of the smell of mown grass.
Preparation: 1-Hexanol is produced through different techniques, for example addition of ethylene to triethylaluminum subsequent oxidation of the alkylaluminum and hydrolysis of the product obtained. It can also be produced by condensation of n-butyraldehyde and acetaldehyde.
Physical properties: 1-Hexanol is a colorless, pleasant smell liquid. Its melting point is -45°C and its boiling point is 157°C. The density is 0.82 g mL-1. It is slightly soluble in water and also soluble in organic solvent as diethyl ether and ethanol.
Chemical properties: 1-Hexanol one of the isomers of 6-carbon alcohols and its chemical properties are directly influenced by the position of the hydroxyl group; as it is at the end of the chain, a long hydrocarbon chain remains at the rest of the molecule, decreasing the solubility of compound in water. Solubility of alcohols in water depends on the hydrogen bond and other polar interactions that can exist between the molecules in water, however, hydrocarbon chain cannot form any polar interaction, depending the solubility only of the hydroxyl group.
Uses: 1-Hexanol is used as an additive in fuels, paints and lubricants. It is largely used in the perfume industry and it is also an intermediate in the preparation of other compounds.
Health effects/safety hazards: It is also highly flammable. It can irritate eyes and skin. 1-hexanol is harmful if swallowed.
|
Related Links: |
