Acetaldehyde Formula
Acetaldehyde, also known as ethanal, is a chemical compound very used in the preparation of perfumes and foods as a flavoring agent.
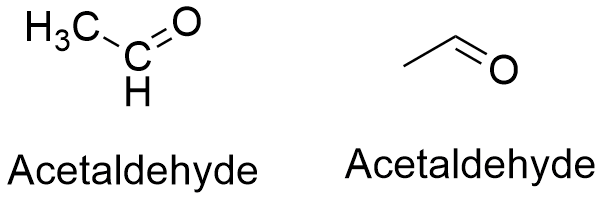
Formula and structure: The acetaldehyde chemical formula is CH3CHO. Its molar mass is 44.053 g mL-1. The acetaldehyde molecule has the usual functional group of an aldehyde H-C=O bound to a methyl group (-CH3), so that the acetaldehyde is the second most simple aldehyde after the formaldehyde. The C atom from the aldehyde has a hybridization sp2, but the methyl group has a sp3, thus the molecule has a planar-trigonal together with a tetrahedral geometry. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Acetaldehyde can be found in many plants and ripe fruits. It is also an intermediate in the ethanol metabolism, through the action of enzymes alcohol dehydrogenase which transform ethanol to acetaldehyde.
Preparation: Acetaldehyde was commonly prepared from the dehydrogenation of ethanol. For that, ethanol reacts over a cooper catalyst at 260-280 ºC.
CH3CH2OH + 1/2 O2 → CH3CHO + H2O
Other method, mostly used today is the hydration of acetylene or ethylene by the Wacker process, which uses a palladium or copper catalyst:
2 CH2=CH2 + O2 → 2 CH3CHO
Physical properties: Acetaldehyde is a colorless, with pungent odor liquid. Its melting and boiling point are -123 ºC and 20.2 ºC and its density is 0.784 g mL-1. Acetaldehyde is soluble is miscible in water, ethanol, benzene, acetone e tolueno. It is slightly soluble in chloroform.
Chemical properties: Acetaldehyde, similar to formaldehyde, is an important precursor in organic synthesis, especially as electrophile. It can s offer condensation reactions to obtain intermediates such as the pentaerythritol (intermediate in the explosive manufacture) that can be used in organic synthesis. It is used to produce hydroxyethyl derivatives through a reaction with a Grignard reagent. Moreover, acetaldehyde is an important building block very used in the synthesis of heterocycles, such as pyridines and imines.
Uses: Acetaldehyde is a chemical compound with a great variety of application in chemical industry. It is largely used in the production of perfumes, dyes, acetic acid and flavoring agent. It Is also used in the fuel composition and like solvent is some industrial process such as the manufacture of rubber, paper and tanning.
Health effects/safety hazards: Acetaldehyde is a very toxic liquid. It is a mucous membranes irritating. Large doses can be fatal due it causes respiratory paralysis. It is highly flammable. May form peroxides. It is a carcinogen suspected compound.
|
Related Links: |
Related Topics
Triethylamine Formula
