Acetylene Formula
Acetylene, also known as ethyne, is a chemical compound of alkyne type that is used as fuel and as intermediate in chemical synthesis.
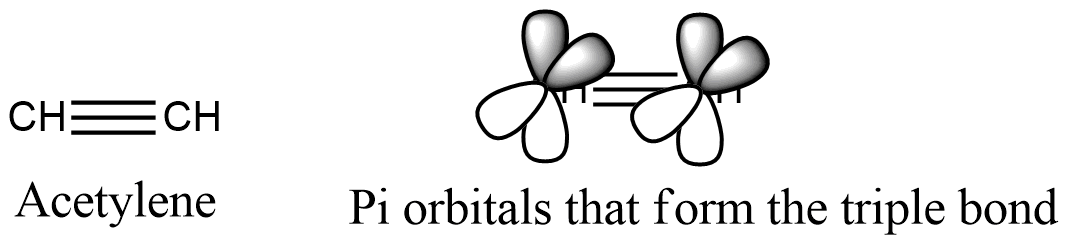
Formula and structure: The acetylene chemical formula is C2H2 and its extended formula is CHΞCH. Its molar mass is 26.04 g mol-1. The molecule is the simplest alkyne, a functional group characterized by having triple bonds. The acetylene molecule is linear (180 ºC), consequently with its carbon atoms hybridized sp. Both carbons has 2 sp orbitals, one bound to the hydrogen and other for the carbons simple bond, while the triple bond, that are 2 Π bonds, is formed between the four orbitals P without hybridization that are orthogonal to the linear system. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Acetylene can be found in nature, especially dissolved in water. It is the main source that use some bacteria to produce acetaldehyde. It is found in natural gas and in oil wells together crude oil and other gases. It is also part of some solar planets atmosphere.
Preparation: Acetylene is recovery from natural gas or oil wells gases but it can also be produced by the combustion or electric arc of hydrocarbons such as methane and ethane or even from the crude oil. This process results in a mixture of gases, so that the acetylene must be separated through other chemical industry process. Other method, less used, is the reaction between calcium carbide and water to produce calcium hydroxide and acetylene.
Physical properties: Acetylene is a colorless gas with garlic-like odor, it is dissolved in acetone to ship. These melting (more correctly triple point because the equilibrium between the three phases) and sublimation points are -80.7 ºC and -84.7 ºC, respectively. Its density is 1.097 g mL-1. It may easily ignite forming a sooty flame. Acetylene is soluble in water, acetone, benzene and chloroform. It is slightly soluble in ethanol.
Chemical properties: Acetylene is a very reactive chemical compound owing its Π electrons in the C-C triple bond, so the acetylene is an excellent nucleophile. Thus, the acetylene can suffer a wide variety of reactions to obtain commercial products, such as acetylide, alcohols, acrylic acid or vinyl compounds. Acetaldehyde can be used to obtain organometallic compounds when reacts with metal as copper.
Uses: Acetaldehyde is widely used by the industry in welding processes owing the high temperature of acetylene flame (3300 ºC). The same flame is used in some lesser development countries as an incandescent lighting. Acetylene can also provide intermediates as ethylene very used in the production of polypropylene by the plastic industry.
Health effects / safety hazards: Acetylene is lighter than air and it is just toxic when produced by methods that can leave other chemical compounds as impurities. Acetylene easily ignited with a sooty flame. It should not be stored together oxidizing agents. It can explode when exposed to fire or heat.
|
Related Links: |
