Ammonium dihydrogen phosphate Formula - Ammonium dihydrogen phosphate Uses, Properties, Structure and Formula
Ammonium dihydrogen phosphate (ADP) is an important member of the ammonium phosphates family, and it is also commonly known as monoammonium phosphate (MAP) or ammonium monophosphate.
Formula and structure: The chemical formula of ammonium dihydrogen phosphate is NH4H2PO4. Its molecular formula is H6NO4P and its molar mass is 115.02 g/mol. It is the mono ammonium salt of phosphoric acid. It is composed of the ammonium cation (NH4+) and the phosphate anion (H2PO4-), as shown in the below chemical structure. The ammonium ion is a common cation which is found in many salts formed by reacting ammonia with an acid. The phosphate anion has the central phosphorous atom attached to two hydroxyl groups and two oxygen atoms (one through single bond and the other through double bond). The solid compound exists as tetrahedral crystals.
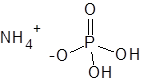
Preparation: Ammonium dihydrogen phosphate is formed by the partial neutralization of phosphoric acid (which has three acidic protons) with one molecule of ammonia. Industrial preparation of ADP involves pumping ammonia gas into an aqueous solution of phosphoric acid, followed by cooling to precipitate the ADP crystals.
NH3 + H3PO4 → NH4H2PO4
Physical properties: ADP is found as a white crystalline solid with a density of 1.80 g/mL and melting point of 190 °C. It is a water soluble solid which is not deliquescent (absorbing moisture from air and turning into solution) unlike other similar compounds.
Chemical properties: Solid ammonium dihydrogen phosphate is stable in air, but when heated, it readily decomposes into ammonia and phosphoric acid. It has fire retardant properties, but reacts explosively in case of sodium fires, or in the presence of sodium bicarbonate.
Uses: Its main application is as a fertilizer due to its being a good source of both nitrogen and phosphate nutrients. It is also used as a dry chemical fire extinguisher and in building materials. It is also found in science kits or crystal growing kits as emerald green, amethyst, or aquamarine crystals.
Health effects/safety hazards: Ammonium dihydrogen phosphate can severely irritate the skin and eyes on contact. It decomposes into ammonia and phosphoric acid, which are both toxic and corrosive materials. Prolonged exposure to ADP can cause ammonia poisoning.
|
Related Links: |
