Ammonium Hydroxide Formula
Ammonium hydroxide, also known as ammonia water or ammoniacal liquor, is an inorganic compound used in cleaning agents.
Formula and structure: The ammonium hydroxide chemical formula is NH4OH. The molar mass is 35.04 g mol-1 respectively. The compound structure is formed by one hydroxide anion (OH-) and one ammonium cation NH4+ which share an ionic bond. Its chemical structure can be written as below, in the common representations used for organic molecules.
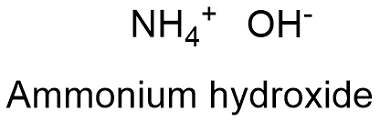
Occurrence: Ammonium hydroxide occurs naturally by the dissolution of ammonia in water.
Preparation: Ammonia hydroxide is formed mostly by the direct reaction of hydrogen and nitrogen catalyzed by a metal as iron. The resulting ammonia is added to water to form the ammonium hydroxide.
Physical properties: Ammonium hydroxide is a colorless liquid with a highly pungent odor. Ammonium hydroxide density is 0.91 g mL-1. The melting points are -57.5 ºC and its boiling point is 37 ºC. It is highly miscible with water.
Chemical properties: Ammonium hydroxide is a weak basic compound that partially dissociates in water keeping the next equilibrium with ammonium ion and hydroxide ion:
NH4OH + H2O ⇌ NH4+ + OH−
The equilibrium is used to regulate the pH of solutions due to the ion OH- increases the pOH or basicity level of a solution.
Uses: Ammonium hydroxide is mostly used as household cleaner, for this purpose it can be used a solution of 1-3%. In chemical processes, it is used as a precursor to form alkyl amines. Ammonium hydroxide is also used as a acidity regulator to reduce the acidity in food due to the release of hydroxide in solution.
Health effects / safety hazards: Ammonium hydroxide is a strong irritant of eyes and respiratory tract. It can also cause severe damage to eyes and skin. It is very toxic to aquatic life.
|
Related Links: |
