Ammonium nitrate Formula - Ammonium Nitrate Uses, Properties, Structure and Formula
Ammonium nitrate is an important inorganic salt that has many uses.
Formula and structure: The chemical formula of ammonium nitrate is NH4NO3. Its molecular formula is written as N2H4O3 and its molar mass is 80.052 g/mol.
Ammonium nitrate is a salt, which consists of two ions: a cation, the ammonium ion (NH4+) and an anion, the nitrate ion (NO3-). The cation and anion are held together by a strong ionic bond.
The structure of ammonium nitrate, composed of the two ions, is shown below:
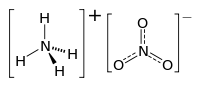
Ammonium cation Nitrate anion
Occurrence: Ammonium nitrate occurs naturally as a mineral in certain desert regions of the world. However, it is not abundant and is found as a mixture with many other minerals. It is easier to prepare synthetic ammonium nitrate, as described below.
Preparation: Ammonium nitrate is commonly prepared by the reaction of ammonia with nitric acid. This is a typical acid-base reaction to give a salt (NH4NO3) as the product.
NH3 + HNO3 → NH4NO3
Physical properties: Its physical state is solid and it typically exists as a white, crystalline or bead-like powder. It has a melting point of 169.6 °C, boiling point of 210 °C, and a density of 1.72 g/cm3.
Chemical properties: Ammonium nitrate is readily water soluble. It is also highly hygroscopic, meaning that it readily absorbs water from the atmosphere and clumps up. It is not particularly reactive and is fairly stable. It decomposes at high temperatures (over 200 °C) to form nitrous oxide and water vapor.
Uses: Ammonium nitrate is most commonly used in fertilizers (as an excellent and inexpensive nitrogen source) and in instant cold packs. It is also used to manufacture explosives as it acts as a strong oxidizing agent. In particular, ammonium nitrate is used to prepare an industrial explosive called ANFO (ammonium nitrate fuel oil), which is composed of 94% ammonium nitrate and 6% fuel oil.
Health hazards/ health effects: While not severely toxic, ammonium nitrate can cause health issues if ingested or inhaled in high concentrations. Ingesting large amounts of ammonium nitrate can cause headache, dizziness, abdominal pain, vomiting, weakness, irregular heart beat and convulsions, while inhalation of this powder may cause breathing problems, coughing, sore throat, and even suffocation at high concentrations.
|
Related Links: |
