Ammonium Sulfide Formula
Ammonium sulfide, also known as azaniumylsulfanylazanium or diammonium sulfide, is an inorganic compound mostly found as a solution used as in photographic developing or in textile manufacturing.
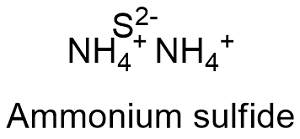
Formula and structure: The ammonium sulfide chemical formula is (NH4)2S and the molar mass is 66.122 g mol-1. The molecule is formed by one centered sulfur atoms to which two ammonium cations NH4+ are attached. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ammonium sulfide is not found in nature as a free compound. It should be prepared as described below.
Preparation: Ammonium sulfide is prepared form the reaction of ammonia and hydrogen sulfide.
HS + exc NH3 → (NH4)2S
Physical properties: Ammonium sulfide is a yellow-orange crystalline solid at temperatures below -18 ºC. It has an unpleasant rotten eggs and ammonia odor. The melting and boiling points are 0 °C and 40 °C, respectively. Ammonium sulfide density is 1 g mL-1. It is soluble with water and ethanol and insoluble in benzene, toluene, hexane and ether. It is unstable at temperatures higher than 0 ºC.
Chemical properties: Ammonium sulfide is commonly found in mixtures with ammonia and hydrogen sulfide due to it suffers dissociation:
(NH4)2S → HS + NH3
Uses: Ammonium sulfide is used to prepare "stink bomb", which is an aqueous dilution of the compound. In water, it decomposes in ammonia and hydrogen sulfide gases which have a unpleasant smell. It is used in photographic developing, as a patina which can be apply to bronze or during some textile processes. It is also used as a reducing agent in some organic synthesis methodologies.
Health effects / safety hazards: Ammonia sulfide can cause eyes, mucous and skin irritation. It is very toxic. It has an unpleasant smell that induces vomits. It is flammable if exposed to fire.
|
Related Links: |
