Barium Chloride Formula
Barium chloride, commonly named as barium muriate or Muryae of Barytes, is an inorganic binary salt used in the fireworks manufacturing.
Formula and structure: The barium chloride chemical formula is BaCl2 and the molar mass is 208.23 g mol-1. This salt is formed by one barium cation (Ba2+) and one chlorine anion (Cl-). Barium salt also has a dihydrate form with a molar mass 244.26 g mol-1. The crystals can be cubic fluorite or orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
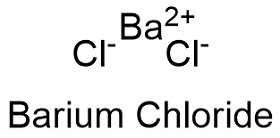
Occurrence: Barium chloride has not been found in nature. It can be obtained by roasting barite with coal and calcium chloride.
Preparation: Barium Chloride can be produced through a two steps reaction from barium sulfate to produce barium sulfate that reacts with HCl to produce barium chloride:
BaSO4 + 4 C → BaS + 4 CO
BaS + 2 HCl → BaCl2 + H2S
It can also be produced from barium sulfide an hydrochloric acid.
Physical properties: Barium chloride is a white solid. Its density is 3.856 g mL-1.The melting and boiling points are 962 °C and 1560 °C. It is soluble in water, methanol and is insoluble in ethanol and ethyl acetate. Its flame is yellow-green but highly toxic.
Chemical properties: Barium chloride has the same behavior or other binary salt of chlorine. In water, it decomposes to form:
BaCl2 → Ba2+ + 2 Cl-
This reaction, also similar to other binary Cl salts as NaCl, does not affect the pH of the solutions.
Uses: Barium chloride is used in the firework industry due to its green flame. It is also used in the treatment of salts produced by heat. It is used in some processes in the petroleum industry. In general, its toxicity limits is uses in laboratory.
Health effects / safety hazards: Barium chloride is extremely toxic. It is toxic by ingestion, inhalation and cause serious eyes damage. It is also toxic to aquatic life. The antidote for a poisonous is the magnesium or sodium sulfate.
|
Related Links: |
