Barium Nitrate Formula
Barium nitrate, also known as Barium dinitrate or barium salt, is an inorganic salt mostly used in the manufacturing of pyrotechnics.
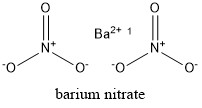
Formula and structure: Barium nitrate chemical formula is Ba(NO3)2 and the molar mass is 261.34 g mol-1. The structure is formed by one barium cation Ba2+ and two nitrate anions NO3-. The crystal structure is cubic with one barium cation surrounded by four nitrate anions. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Barium nitrate can occur naturally in the mineral nitrobarite. However, the quantities in which it is found are too low and instead, it should be prepared as described below.
Preparation: Barium nitrate is prepared from the reaction of nitric acid with barium metal or with the reaction of nitric acid and barium oxide:
2HNO3 + Ba → Ba(NO3)2 + H2
2HNO3 + BaO → Ba(NO3)2 + H2O
Additionally, it can be prepared dissolving barium carbonate in nitric acid.
Physical properties: Barium nitrate is a white lustrous crystalline solid. Its density is 3.24 g mL-1. The melting point of the salt is 592 °C and above this temperature decomposes. Barium nitrate is highly soluble in hot water and poor soluble cold water. It is not soluble in ethanol. It burns with a green flame.
Chemical properties: Barium nitrate is a strong oxidizing agent, thus it can oxide some metal and oxide and react violently with them. Due to its high content of nitrate, it is used for military purposes to produce grenades and other explosives. At high temperatures, it can decompose to form barium oxide and NO2:
2 Ba(NO3)2 → 2BaO + 4NO2 + O2
Uses: Barium nitrate is used in the production of pyrotechnics, where is the compound responsible by the green color observed in the flames. It also is used in the production of some materials that contains mixtures of barium oxide. This nitrate was used as a component of some explosives as the Baratol, with also included the TNT in its formulation. Today it is still used along with the aluminum powder in some explosives as Thermate-TH3.
Health effects / safety hazards: Barium nitrate is toxic by ingestion or inhalation. It can also cause eyes and respiratory tract irritation. In contact with aluminum powder, is formed a very explosive powder. It is a strong oxidizing agent that can react violently with other metal oxides.
|
Related Links: |
