Barium sulfate Formula - Barium sulfate Uses, Properties, Structure and Formula
Barium sulfate is an important inorganic chemical with many applications, including medical uses.
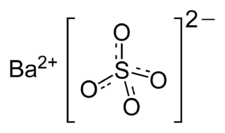
Formula and structure: The chemical formula of barium sulfate is BaSO4 and its molar mass is 233.43 g/mol. It is a salt composed of the barium cation (Ba2+) and the sulfate anion (SO42-), in which sulfur is attached to four oxygen atoms. The barium metal is in the +2 oxidation state. The chemical structure of barium sulfate is shown below:
Occurrence: Barium sulfate occurs naturally as the mineral barite, which is widely found and used as the major source of barium and other barium compounds.
Preparation: Barium sulfate is obtained in commercial amounts from the mineral barite, after mining and processing. The processing of the impure barite involves heating it with coke (carbon) to form the water-soluble barium sulfide (BaS), which is then separated from the impurities and reacted with sulfuric acid to give the pure barium sulfate product:
BaSO4 + 4 C → BaS + 4 CO
BaS + H2SO4 → BaSO4 + H2S
Another method to obtain pure barium sulfate is by reacting barium carbonate or barium chloride with sulfuric acid.
Physical properties: Pure barium sulfate is found as white, odorless powder or small crystals with a density of 4.49 g/mL, melting point of 1580 °C and boiling point of 1600 °C.
Chemical properties: Barium sulfate is known for its poor solubility in water. It is also insoluble in alcohols, and soluble in concentrated acids. It reacts violently with aluminum powder. Barium sulfate has several medical and radioimaging uses due to its water insolubility and radio-opaque properties.
Uses: Barium sulfate is widely used as a radio-opaque agent or X-ray contrast agent to diagnose gastrointestinal medical conditions. It is also used in oil well drilling fluids, and concrete-based radiation shields. It also has applications in root canal fillings, pigments, paints, adhesives, paper coatings, fillers in plastics, linoleum, brake linings, pyrotechnic compositions, textiles, rubber and catalyst supports.
Health effects/safety hazards: Barium sulfate is considered nontoxic due to its being insoluble in aqueous media and is safe for medical uses. However, exposure to its dust or inhalation in high concentrations can cause some irritation to the eyes, nose, and respiratory system.
|
Related Links: |
