Calcium phosphate Formula
Calcium phosphate is an inorganic salt found in minerals and ores that is mostly used in the production of fertilizers and phosphoric acid.
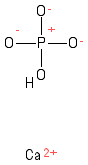
Formula and structure: The calcium phosphate chemical formula is CaH4P2O8. The molar mass is 234.05 g/mol. Calcium phosphate can be referred to a several combination of the cation calcium Ca2+ and the anion phosphate PO43-. For example, it exists monocalcium phosphate with the formula Ca(H2PO4)2 or the tricalcium phosphate with the formula Ca3(PO4)2. In this article, we mostly comment about the most common dicalcium phosphate CaHPO4. It can also be found as a dihydrate salt CaHPO4.2H2O. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Calcium phosphate is found in nature. It can be found in minerals and soils. Calcium phosphate is also found in bone and tooth, to which arrives via consuming of milk. Milk contains calcium phosphate in the form of micelles.
Preparation: Calcium phosphate can be prepared performing a reaction between the calcium hydroxide and phosphoric acid:
Ca(OH)2 + 2 H3PO4 → Ca(H2PO4)2 + 2 H2O
It can also be produced by the reaction at high temperature of phosphate rock and calcium oxide.
Physical properties: Calcium phosphate is a white powder odorless. The density of this salt is 2.22 g/mL. Its melting point is 109 °C and above the temperature of 203 °C, it decomposes. It is slightly soluble in water, with only 2 g/ 100 mL of solubility. It is insoluble in organic solvent.
Chemical properties: Calcium phosphate is stable and versatile in the reaction that can perform. Thus, it can be used to react with other chemical compounds in different households products. One of these examples is the mixture of calcium phosphate with sodium bicarbonate to create a leavening agent. When the calcium phosphate is in contact with a weak base as the bicarbonate, it forms carbon dioxide and a binary salt.
Uses: Calcium phosphate is used as fertilizer and can also be used as raw material in the production of more sophisticated fertilizers such as the superphosphate. It is also a material used in the manufacture of glass, dental powders and porcelains. Calcium phosphate can be used in the textile and food industry as a stabilizer. Some uses as a pigment are also known.
Health effects / safety hazards: Calcium phosphate is an irritant by inhalation. It can irritate the respiratory tract and mouth. It is not flammable and it does not react with other chemical compounds.
|
Related Links: |
