Chloroform Formula
Chloroform, also known by the IUPAC name of trichloromethane, is an organic solvent very used in chemical and pharmaceutical industries. In the past, it was used as anesthetic.
Formula and structure: Chloroform chemical formula is CHCl3 and its molar mass is 119.37 g mol-1. The molecule has the typical structure of a methane, which is the most related molecule due to chloroform is a methane where 3 hydrogen atoms has been substituted by 3 chloride atoms. Thus, the structure of the chloroform is a tetrahedral. Its chemical structure can be written as below, in the common representations used for organic molecules.
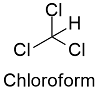
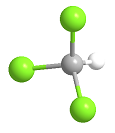
Occurrence: Chloroform is found in nature as being produced by some fungi and bacteria in soils and seas.
Preparation: Even when chloroform can be found in nature, the total of the solvent used by the industries is synthesized through chemical methods. The first method is the chlorination of the methane or chloromethane until chloroform, and given dichloromethane as side compound. A second strategy is the reaction between a solvent as acetone or ethanol and a chlorinate lime.
Physical properties: Chloroform is a colorless liquid with and ethereal odor. Its density is 1.489 g mL-1 and its melting point is -63.5 ºC and its boiling point is 61.15 °C. It is slightly soluble in water and it is soluble in benzene, ditethylether and carbon tetrachloride.
Chemical properties: Chloroform is a non-polar solvent due to the molecule has three chlorine atoms (which have a high electronegativity) but these are orientated in opposite directions, thus when the moment dipolar total is low. Moreover, the chloroform is low reactive and thus, it can be used as solvent in many reaction in organic chemistry.
Uses: Chloroform is today largely used as solvent in chemical processes such as the production of Teflon, where chloroform is used to obtain monochlorodifluormethane, a precursor in the process:
CHCl3 + 2 HF → CHClF2 + 2 HCl
Moreover, chloroform is used in the synthesis of several pesticides, plastics, resins, etc. And its properties as solvents are particularly effective to extract natural compounds form plants, for example some alkaloids such as morphine. For many years, chloroform was used as anesthetic until it was confirmed as a carcinogenic agent.
Health effects / safety hazards: Chloroform is extremely harmful when swallowed or inhaled. It can cause irritation in skin and eyes. The compound can also cause dizziness and it is suspected to cause cancer and infertility.
|
Related Links: |
