Condensed structural Formula
Definition: the condensed structural formula is a system of writing organic molecules in shorthand notation with more details that the molecular formula but lesser extended that the normal structural formula.
The condensed formula is written in just one line of text and it lists the atoms in the order that they occupy in the molecule, etc. It also shows the functional groups that are presents in the molecule, such as amine -NH2, alcohol -OH and parenthesis are used for showing that polyatomic groups are part of the same chain. For example, the condensed structural formulas of methane and propanol are CH4 and CH3(CH2)2OH.
Formula: It is important to distinguish between the three types of formulas in organic chemistry for avoiding confusions.
-
Molecular formula (shows the number of atoms of each element) C5H12O
Condensed structural formula (shows all the atoms but admit vertical bonds and polyatomic groups are inside parenthesis) CH3(CH2)4OH
Structural formula (shows all the bonds)
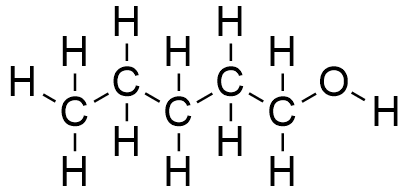
Use: It is extremely useful for showing the functional groups of a molecule. The functional groups are the responsible for giving the chemical and physical characteristics to all the molecules, so that, knowing the number and type of functionality of these groups, a chemist can estimate the behavior of compounds. Moreover, condensed structural formula also shows the geometry of some molecules and it is also a very important characteristic in the attribution of the property to chemical compounds.
Example: The molecular formula of a unknown alcohol is C3H8O, write three of the possible condensed formular of this compound.
C3H8O → isopropanol (CH3)2CHOH → 2-propanol CH3CHOHCH3 → 1-propanol CH3OHCH2CH3
Considerations: The condensed structural formula is important for describing cases as the described above, in which the same molecular formula can lead to more of one structure. Structure that have the same number of atoms and mass weight but with different functional groups or with the same but in different positions, are called isomers.
|
Related Links: |
