Ferric sulfate Formula
Ferric sulfate is an inorganic salt, which is used in several chemical application such as water purification, in medicine as an astringent and as pigment and deoxidize.
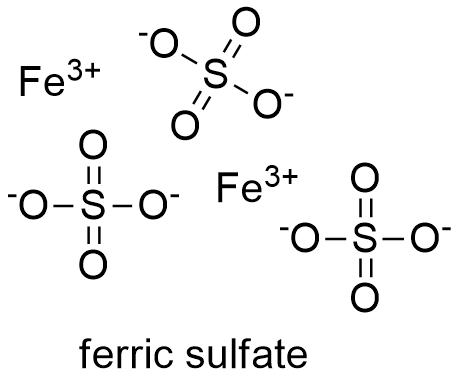
Formula and structure: The ferric sulfate molecular formula is Fe2(SO4)3. Its molar mass is 399.88 g mol-1. The ferric sulfate structure contains Fe+3 cations and SO4-2 anions, which form a crystalline salta with rhomboidal geometry. Ferric sulfate also has some hydrated forms; for example: the nonahydrate or heptahydrate ferric sulfate. The chemical structure for the anhydrous ferric sulfate can be written as below, in the common representations used for organic molecules.
Occurrence: The anhydrous ferric sulfate is not common in nature. The nonahydrate, hexa- and penta- form are found in major quantity in nature, however these are also rarely to find. Ferric sulate (anhydrous and hydrated forms) are very unstable, thus these compounds tend to form bond with chemical compounds, in order to be in a more stable mineral, like the case of iron-aluminium sulfate.
Preparation: Ferric sulfate is prepared in industry by boiling concentrated sulfuric acid into ferrous sulfate or ferric hydroxide. Other methods use chlorine gas instead sulfuric acid. It is also added an oxidizing agent as hydrogen peroxide to the reaction.
2FeSO4 + H2SO4 + H2O2 → Fe2(SO4)3 + 2H2O
Physical properties: Anhydrous ferric sulfate is a yellow crystalline salt or a grayish-white powder. Its melting point is 480 ºC and its density is 3.097 g mL-1. It is slowly soluble in water and insoluble in acetone and ethyl acetate.
Chemical properties: Ferric sulfate hydrolyzes slowly in solution and it is insoluble in sulfuric acid, thus this acid can be used to prepare ferric sulfate industrially. This salt decomposes when heated.
Uses: Ferric sulfate is mainly used to treat and purify water due its capacity to coagulant industrial wastes. It is used in bleaching and dyeing textile manufacture. Ferric sulfate can also be an oxidizer or reducer agents. Moreover, ferric sulfate has medicinal application as astringent and styptic.
Health effects/safety hazards: Ferric sulfate is an irritant substance when in contact with the skin and eyes. It also irritates the nose and throat and stomach by ingestion.
|
Related Links: |
Related Topics
Normality Formula
