Hydrofluoric Acid Formula - Hydrofluoric Acid Uses, Properties, Structure and Formula
Hydrofluoric acid (HF) is the aqueous solution of hydrogen fluoride. It is also called fluoric acid or fluorhydric acid.
Formula and structure: The chemical formula of hydrofluoric acid is HF. Its molar mass is 20.01 g/mol. Hydrogen fluoride is the gaseous form, while hydrofluoric acid is the solution of HF in water. HF is a simple diatomic molecule with the below structure:

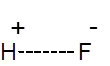
Hydrogen fluoride is polarized due to the electronegativity of the fluoride, allowing the H+ to be easily dissociated (thus making it an acid).
Preparation: The industrial preparation of hydrofluoric acid involves the reaction of fluorite (CaF2) with concentrated sulfuric acid at a high temperature (265 °C). This reaction produces hydrogen fluoride and calcium sulfate as shown below:
The hydrogen fluoride gas is then dissolved in water to prepare the hydrofluoric acid, in different concentrations.
Physical properties: Hydrogen fluoride is a colorless gas with a density of 1.15 g/L at room temperature, or a colorless liquid (below 20°C) with a density of 0.99 g/mL. Hydrofluoric acid (solution of HF in water), is a colorless solution. Its exact physical properties (boiling point, melting point and density) depend on the concentration of HF in the aqueous solution.
Chemical properties: Hydrofluoric acid is a very strong, reactive and corrosive acid. It readily reacts with bases, acids, and oxidants. One of its best known reactions is its corrosive, dissolving effect on glass and ceramics (called etching). Due to reactivity towards glass and metals, it is typically stored in plastic containers.
Uses: Hydrofluoric acid is widely used in the preparation of many useful fluorine compounds, such as Teflon (PTFE plastic), Freon (refrigerant), fluorocarbons, and many medications such as fluoxetine (Prozac). It is also used for many industrial purposes such as glass etching, metal cleaning, and rust removal. It is used in the semiconductor industry to clean silicon wafers.
Health hazards/ health effects: Hydrofluoric acid is a highly corrosive liquid and a contact poison which can cause severe poisoning through skin contact, inhalation and ingestion. HF rapidly penetrates tissues, and can permanently damage eyes, lungs, and mucous membranes. Upon skin contact, the concentrated acid also causes severe burns and can even lead to cardiac arrest and fatality. Even mild HF exposure can cause symptoms such as irritation of the eyes and throat, eye and skin burns, pulmonary edema and bone damage.
|
Related Links: |
