Ionic strength (ionic strength formula)
Definition: Ionic strength is a measure of the ions concentration ions solution. It was defined by Lewis and Randall in 1921 and it is based on the dissociation that suffers salts, acid and bases when are in an aqueous solution. It is expressed in concentration units, such as molar concentration (mol/L).
General formula: The ionic strength formula is calculate as the sum of the molar concentration of each ion multiplied by the valence squared.
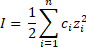
where the term 1/2 is due to both ions are considered (cation and anion), c is the concentration in molar units (mol/L) and z is the charge of each ions. For example, if the ions is sulfate (SO 42-) z = 2. This, it can be seen multivalent ion has a bigger contribution.
Use: It is used in theoretical chemistry for calculating dissociation of salts in heterogeneous systems such as colloids. It is also used in biochemistry and molecular biology for determining the strength of buffer solutions that should have concentrations similar to the found in nature.
Example: For a solution of potassium chloride 3 M, calculate the ionic strength.
First, it is needed to draw the dissociation: KCl → K+ + Cl-
so, the concentration of each ion is the same as the concentration of the salt, 3 mol/L.
Then, the equation can be applied:
I = 1/2 [(3 mol/L)(+1)2 + (3 mol/L)(-1)2] = 3 M
For a solution of potassium chloride 1 M and magnesium sulfate 0.2 M, calculate the ionic strength.
First, it is needed to draw the dissociation: KCl → K+ + Cl- and MgSO4 → Mg2+ + SO42-
Then, the equation can be applied:
I = 1/2 [(1 mol/L)(+1)2 + (1 mol/L)(-1)2 + (0.2 mol/L)(+2)2 + (0.2 mol/L)(-2)2 ] = 3.6 M
Considerations: For non-ideal solutions (solutions that non follow the ideal behavior, for example solutions where the interaction forces should be considered), it is used the unit of molality (mol/kg of H2O) instead of molarity.
|
Related Links: |
