Iron II Phosphate Formula
Iron (II) phosphate, also known as ferrous phosphate, is an inorganic salt of iron and it is a component of fertilizers and herbicides.
Formula and structure: The iron (II) phosphate chemical structure is a Fe3(PO4)2 and its molecular weight 357.448 g/mol. It is structure is monoclinic and in general, it is formed by three cations Fe 2+ and two anions PO43- that are surrounded by eight water molecules. It is commonly presents in its hydrated form Fe3(PO4)2 · 8H2O. Its chemical structure can be written as below, in the common representations used for organic molecules.
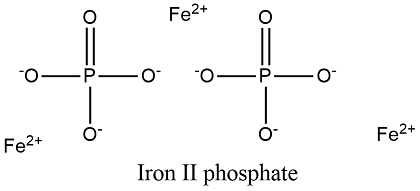
Occurrence: Iron (II) phosphate, similar to other metallic phosphates, is found in many minerals. One of these is the mineral vivianite, in which the hydrate form of ferrous phosphate is found.
Preparation: It is produce by the reaction of ferrous hydroxide with phosphoric acid, yielding a precipitate of ferrous phosphate.
Fe(OH)2 + H3PO4 → Fe3(PO4)2(s)
Ferrous phosphate can also be produced as a precipitate from the reaction of ferrous chloride and sodium or potassium phosphate.
3 FeCl2 + 2 Na3PO4 = Fe3(PO4)2(S) + 6NaCl
3 FeCl2 + 2 K3PO4 = Fe3(PO4)2(S) + 6KCl
Physical properties: Ferrous phosphate is a odorless, brown powder. Its density is 2.61 g/mL and its melting point is 180 ºC, that represents is decomposition. It is not soluble in water but it is soluble in acid solutions.
Chemical properties: Ferrous phosphate has the capacity to avoid the oxidation of metal, thus it is used added to steel or used as metallic rustproofing. Different to other metallic component, it has a low electronic conductivity; however, this characteristic allows its use for specific electronic purposes. It is also considered a thermal stable compound.
Uses: Iron (II) phosphate is used as a component of farming products. It is used in herbicides and molluscicides. It is also used in coatings for paints, fabrics and other materials as wood. Moreover, iron (II) phosphate is used in the electronic industry as a component for producing batteries in electric cars.
Health effects/safety hazards: Iron (II) phosphate can cause eye, skin and respiratory tract irritation. It is dangerous if swallowed or inhaled. It is not flammable.
|
Related Links: |
