Lead (II) azide Formula
Lead (II) azide, also known as diazidolead, is an inorganic compound largely used in the explosives industry to produce detonators.
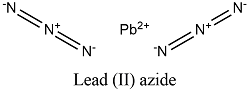
Formula and structure: The lead (II) azide chemical formula is Pb(N3)2 and its molar mass in 291.242 g mol-1. The molecule is formed by the lead (II) cation, Pb+2 and 2 azide anions N3-. The anion azide is formed 3 nitrogen atoms which are bound by 2 double bonds; the anion also has two partial negative charges and one partial positive charge: -N-=N+=N--. The lead (II) azide structure is orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Lead (II) azide is very instable, so that it is not found in nature. It was synthesized by the first time in the 19th century and in the 20th century was developed alternative methods to produce it easily and cheaper to use in war.
Preparation: Lead (II) azide is produce from the reaction between lead nitrate or lead acetate and sodium azide. The reaction is prepared in aqueous solution and in basic conditions; moreover, the size of the crystal must be controlled since the larger crystal, the higher risk to produce explosions.
Pb(NO3)2 + 2 NaN3 → Pb(N3)2 + 2 NaNO3
Physical properties: Lead (II) azide is an odorless, white powder or needles. Its density is 4.70 g mL-1. The melting point is 190 ºC and in temperature higher than 300 ºC, lead (II) can explode. It can also explode from flame or friction. It is insoluble in water and ammonium hydroxide but is soluble in acetic acid.
Chemical properties: Lead (II) azide is formed by the anion azide N3-, which is largely known as a component of air bag and propellants; this anion is linear and has several structures of resonance. The instability of the ion azide is increased when forms metallic salt as the lead azide, moreover, with metal as copper or zinc the resulting salt are even more instable.
Uses: Lead (II) azide has been used since the late 19th century as explosive or as component on the production of many explosives.
Health effects / safety hazards: Lead (II) azide is extremely harmful if swallowed and may also cause damage to organs and eyes. It can also affect fertility or damage in the unborn children. It is very toxic to aquatic life. Lead (II) azide is too unstable, so that there is serious risk of violent explosions.
|
Related Links: |
