Lead (II) sulfide Formula
Lead sulfide or sulphide, also known as galena or plumbous sulfide, is an chemical compound used in electronic industry to produce semiconducting materials.
Formula and structure: Lead sulfide chemical formula is PbS and its molar mass is 239.26 g mol-1. Lead sulfide is formed by the cation Pb+2 (the lesser oxidized ion of Pb) and the anion S-2 (the lesser oxidizer ion of S). Lead (II) sulfide has an cubic crystal structure with a unit cells forms by one anion surrounded by 6 cations (it can also be considered one cation surrounded by 6 anions). Its chemical structure can be written as below, in the common representations used for organic molecules.
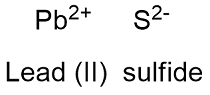
Occurrence: Lead (II) sulfide is found in geological formations, particularly, in mineral ores called galena. It is also found in the outsider space, for example, on the planet Venus.
Preparation: Lead (II) sulfide is extracted from the galena ores, however, there are some chemical methods to prepared this inorganic compound. It has been mainly prepared by the reaction of substitution between hydrogen sulfide gas and lead nitrate solution.
H2S + Pb(NO3)2 → PbS + 2 HNO3
Physical properties: Lead (II) sulfide is a black crystalline solid or a silver powder. Its density is 7.5 g mL-1. Its melting point is 1114 ºC and its boiling point is 1281 ºC. It is insoluble in water and soluble in nitric acid hot and hydrochloric acid.
Chemical properties: Lead (II) sulfide is an semiconductor, photoconductor and infrared radiation material. Its capacity is owing the lead atom is a post-transition metal elements, placed in the group 14 and period 6 of periodic table between the metals and metalloids elements, so that its metallic character is weak and has some characteristics of metalloids, such as the semi- or photo- conductivity. Moreover, the lead sulfide is a very inert compound, which very useful to produce electronic components.
Uses: Lead (II) sulfide has been used during many years as source of lead (Pb). The main method to obtain the lead is the smelting of PbS and then, the lead (II) oxide obtained is reduced to Pb and carbon monoxide:
2 PbS + 3 O2 → 2 PbO + 2 SO2
PbO + C → Pb + CO
Moreover, lead (II) sulfide is used as semiconductor and photoconductor due its chemical proprieties. It is also used as black pigment. In recent years, it has been used in to obtain nanoparticles to use in electronic or electric devices.
Health effects / safety hazards: Lead (II) sulfide is dangerous by inhalation or ingestion. It can cause strong eye irritation and corneal destruction. It is not flammable. It emits very toxic vapors when heated.
|
Related Links: |
