Lythium hydroxide Formula
Lithium hydroxide is an inorganic basic compound. It is largely used in organic synthesis to promote reaction due its strong basicity.
Formula and structure: The lithium hydroxide chemical formula is LiOH and its molar mass is 23.91 g mol-1. It exists in two forms: the anhydrous and the monohydrate LiOH.H2O, which has a molar mass of 41.96 g mol-1. In general, the lithium hydroxide molecule is formed by the lithium cation Li+ and the hydroxyl group OH-. The lithium hydroxide is the only alkali hydroxide that does not present polymorphism, and its lattice has a tetragonal structure. Its chemical structure can be written as below, in the common representations used for organic molecules.
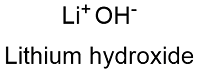
Occurrence: Lithium hydroxide is not found freely in nature. It is very reactive and if it would be in nature could react easily to form other compounds. However, some Lithium/aluminium hydroxides forming diverse mixtures can found in mineral ores.
Preparation: Most of the lithium hydroxide is produced from the reaction between lithium carbonate and calcium hydroxide. This reaction yields lithium hydroxide and also calcium carbonate:
Li2CO3 + Ca(OH)2 → 2 LiOH + CaCO3
It is also prepared from the reaction of lithium oxide and water:
Li2O + H2O → 2 LiOH
Physical properties: Lithium hydroxide is white, hygroscopic solid with a pungent odor. Its density is 1.46 g mL-1 in the anhydrous salt and 1.51 g mL-1 in the monohydrate. The melting and boiling points are 462 ºC and 924 ºC, respectively. It is poorly soluble in water, ethanol and methanol and insoluble isopropanol.
Chemical properties: Lithium hydroxide and the other alkalis hydroxides (NaOH, KOH, RbOH and CsOH) are very versatile to use in organic synthesis because are stronger bases which react easily. It may react with water and carbon dioxide at room temperature. It can also react with many metals as Ag, Au, Cu and Pt, so that it has been an important starting material in organometalic synthesis.
Uses: Lithium hydroxide is largely used to produce soaps, greases and lubricating through the esterification of fat promoted by the LiOH basic character. It has been used to absorb carbon dioxide in submarines, spacecraft and in the scrubbing equipments. Lithium hydroxide and other lithium compounds have been used recently to development and studied new type of batteries.
Health effects / safety hazards: Lithium hydroxide contact can cause serious damage to eyes, respiratory system and skin. It may be extremely toxic by ingestion, skin absorption and inhalation. It can react violently with water. When heated can produce toxic fumes.
|
Related Links: |
