Mercury (II) chloride Formula
Mercury (II) chloride, also known as mercury bichloride, is an inorganic salt that is still used in many disinfectants and in photography materials. It is highly toxic.
Formula and structure: The mercury (II) chloride chemical formula is HgCl2 and its molar mass is 251.72 g mol-1. The molecule is formed by a mercury (II) cation Hg+2 and a two chloride anions Cl-1, which form two ionic binds. The crystal structure is orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
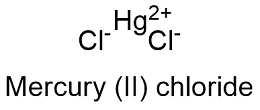
Occurrence: Mercury (II) chloride is not produce by nature or biologic system, it is only produced by chemical processes.
Preparation: Mercury chloride is synthesized through several methods such as the reaction between mercury (I) chloride and chlorine pure or also through the reaction between the hydrochloric acid and mercury (I) nitrate at 300 ºC. Additionally, it can be produced by the mixture between mercury (II) sulfate and sodium chloride at high temperatures and on dry atmosphere.
Physical properties: Mercury (II) chloride is a colorless to white crystalline solid. Its density is 5.43 g mL-1. Its melting point is 276 ºC and its boiling point is 304 ºC. Mercury (II) chloride is poorly soluble in cold water but soluble in hot water, ethanol, ethyl acetate and acetone. It is slightly soluble in pyridine and benzene.
Chemical properties: Although mercury (II) chloride is extremely toxic, it is still used in many industries such as the metallurgic due to it can form amalgams with other metals such as aluminium. In the century XIX was used as a material in the railroad building but it was not use anymore because its toxicity.
Use: Mercury (II) chloride is mostly used as catalyst for both: organic and inorganic synthesis. It was used many year in the treatment of syphilis and other illnesses but now, it is not used as medicine because its toxicity. Moreover, mercury (II) chloride is used as reagent to reveal photos and in the preservation of biological samples.
Health effects / safety hazards: Mercury (II) chloride is extremely toxic by ingestion or inhalation. It is fatal when in contact with skin and cause severe eyes damage. It is also a suspected mutagenic agent and can also cause fertility problems. It is toxic to aquatic environment. It is not flammable, but is incompatible with strong acids, ammonia, metallic salts and carbonate.
|
Related Links: |
