Molar concentration (molar concentration formula)
Definition: Molar concentration, also known as molarity, is a way to express the concentration of a solute in a solution. It measures the quantity of substance per unit volume. Specifically, it expresses the mole of a substance per litre of solution. The symbol is M or mol/L.
General formula: The molar concentration is calculated as the mol of a substance in 1 L of solution, thus:
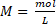 , it is also possible to use the mass in gram of the substance and the conversion to mole is made using the molecular weight (MW):
, it is also possible to use the mass in gram of the substance and the conversion to mole is made using the molecular weight (MW):
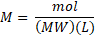
A solution with a salt concentration of 1 M has 1 mol of solute in 1 L of solution.
Use: Molarity is one of the most used units in laboratory. Its use is routinely and almost all the solutions used for preparing chemical substances or for other uses are expressed in molar concentration units. Many times, it is also used for expressing the amount of substances in physical chemistry analysis, especially in food and pharmaceutical industries.
Example: 10 g of KCl is dissolved in 250 mL of distilled water, calculate the molar concentration.
First, the molecular weight of KCl is 74,55 g/mol, then the formula can be applied:
[M] = (10 g / (74.55 g/mol * 0.25 L) = 0.54 M
Notice that, the volume was converted from mL to L and the symbol [M] means concentration.
How many gram of NaBr are needed for preparing 100 mL of a solution 0.5 M in water?
The Molecular weight of NaBr is 102.89 g/mol. The sme formula allows making this calculate:
mol = M*L and g = mol*MW
mol NaBr = 0.5 M*0.1 L = 0.05 mol and g NaBr = 0.05 mol*102.89 g/mol =5.15 g NaBr
Considerations: The molar concentration follow the same rules that the other units in the International System of Units, so that is possible to use the prefix mili, micro, nano and others for describing 10-3mol, 10-3mol and 10-3 mol.
|
Related Links: |
