Nitrite Formula
Nitrite is an inorganic ion with a negative net, which is a component of many salts as sodium nitrite or potassium nitrate. These salts are largely used in the food industry.
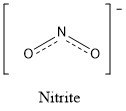
Formula and structure: Nitrite ion chemical formula is NO2− and it molar mass is 46.00 g mol-1. It is formed by a central nitrogen anion that is joined to two oxygen atoms through to covalent bonds with the same distance. One negative charge is shared between the two oxygen atoms through a resonance effect. The nitrite ion has a symmetrical structure, forming a inverted V of 115º between the central N and the two oxygen atoms. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: This anion is not found in nature as free specie.
Preparation: Nitrite salts can be prepared from different methodologies depending on the cation that is bond to the specie. For example, the sodium nitrite is prepared through the reduction of nitrate salts with a catalyst in basic media or by the oxidation of other nitrogen oxides.
Physical properties: Nitrite is a ion that can be found as part of some salts as sodium or potassium nitrite, so it can have different physical properties according to the cation that is bond to. In the case of sodium nitrite is a white crystalline solid with a melting point equals to 271 ºC and with a decomposition temperature above 320 ºC. The density for this salt is 2.17 g mL-1. In the case of potassium nitrite, it is a white deliquescent solid with a melting and boiling points of 440 ºC and 537 ºC.
Chemical properties: The nitrite ion is unstable under some conditions. For example, sodium nitrite that is ingested by humans can suffer a nitrosation, forming species that are classified by the health agencies as probably carcinogenic. Also related to the food uses of nitrite, it can react with the myoglobin presents in red meat, forming red compounds as the nitromyoglobin and lately, when heated (cooking) nitrosohemochrome.
Uses: Nitrite is used in the form of salt as an additive in meat curing. Despite of this possible carcinogenic effect, it has been used for many years due to the prevention of botulism. It is also used as bactericide due to potassium and sodium nitrite can help to inhibit some micro-organisms such as clostridia. Nitrite is also very used as in inorganic synthesis because it can act as an ambidentate ligand.
Health effects / safety hazards: Nitrite is a strong oxidizing agent that can react violently with other substances. It is also classified as a possible carcinogenic.
|
Related Links: |
