Phosphorous trichloride Formula
Phosphorous trichloride, also known as phosphorus (III) chloride, is a chemical compound mainly used in the production of organophosphorus substance for chemical industry.
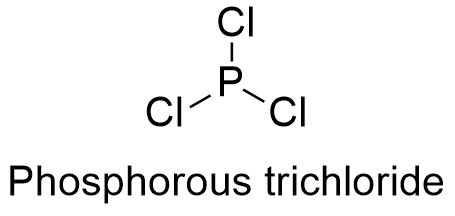
Formula and structure: The phosphorous trichloride chemical formula is PCl3 and its molar mass is 137.33 g mol-1. The molecule is formed by the cation phosphorous and 3 anions chloride. The molecule has a geometry trigonal pyramidal due the repulsion caused by a lone pair of the phosphorous under the 3 chloride atoms. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Phosphorous trichloride is not found in nature. It is just obtain through organic synthesis.
Preparation: Phosphorous trichloride is mostly produced from the yellow or white phosphorous and gas chlorine. The reaction also produces phosphorous pentachloride,however in order to avoid the formation of PCl5 is added more phosphorous:
P4 + 6 Cl2 → 4 PCl3
Physical properties: Phosphorous trichloride is a yellow to colorless, liquid, with pungent and irritating odor. Its melting and boiling point are -93.6 ºC and 76.1 ºC and its density is 1.574 g mL-1. Phosphorous trichloride reacts with water and ethanol and it is soluble benzene, carbon tetrachloride, chloroform and ether.
Chemical properties: Phosphorous trichloride has an interesting chemical behavior. It can suffer many reactions and being a electrophile and nucleophile for promoting a great variety of reactions. It can react through redox reactions to form other phosphorous compounds such as pentachloride, phosphoryl chloride or thiophosphoryl chloride. Because the lone pair of phosphorous it can act as nucleophile in some reactions, especially with Lewis acids as AlCl3. Moreover, the phosphorous can accept more electrons, so that it can also be an electrophile.
Uses: Phosphorous trichloride is used widely in chemical industry, mainly in the production of pesticides, surfactants, gasoline additives, plastics, medicinal products and textiles. It is also used in the production of other phosphorous compounds such as phosphorous pentachloride, phosphoryl chloride or thiophosphoryl chloride. Moreover, phosphorous trichloride is also used as precursor and intermediate of some chemical reactions as Wittig reaction.
Health effects/safety hazards: Phosphorous trichloride can cause severe damage to skin, eyes and mucous membranes. It is fatal by ingestion, inhalation and can be absorbed by skin. Additionally, this compounds reacts violently with water producing irritating fumes which are extremely toxic. It is not flammable.
|
Related Links: |
Related Topics
Nitrous Acid Formula - Nitrous acid Uses, Properties, Structure and ...
Sodium chloride Formula - Sodium chloride Uses, Properties ...
