Potassium dichromate Formula
Potassium dichromate is an inorganic salt commonly used as astringent and antiseptic to external application due it is also a poison.
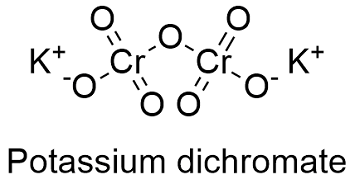
Formula and structure: The potassium dichromate chemical formula is K2Cr2O7 and its molar mass is 294.185 g mol-1. The molecule is formed by 2 potassium cations K1+ and 1 dichromate anion Cr2O72-. It is found in both form: the anhydrous and the three hydrate. Its lattice structure is triclinic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sodium dichromate is naturally formed in the mineral lopezite which can be found in deposits in South America and Africa.
Preparation: Sodium dichromate is prepared through the reaction of potassium chloride and sodium dichromate. It can also be prepared with potassium chromate and potassium hydroxide.
2 KCl + Na2Cr2O7 → K2Cr2O7 + 2 NaCl
Physical properties: Potassium dichromate is a red or orange and ordorless crystalline solid. Its density is 2.676 g mL-1 and its melting and boiling points are 398 ºC and 500 ºC. Potassium dichromate is soluble in water. It is insoluble in ethanol.
Chemical properties: Potassium dichromate is used largely in chemical industry and laboratory due the dichromate ion is a powerful oxidant. The chrome is easily reduced from Cr (VI) to Cr (III). This reaction is easily observable because the Cr (VI) is orange and Cr (III) is green and it is used in analytical chemistry to quantify or determinate the presence of hydrogen sulfide or ferrous ions. It is also used in organic chemistry to oxidize alcohols to aldehydes or ketones.
Uses: Potassium dichromate is still largely used in chemical industry and laboratories to promote oxidation reactions, however in the last years its use has decreased due potassium dichromate is highly toxic. Moreover, potassium dichromate is used as preservative for wood. It is also used to manufacture pigments and coats and in leather tannig.
Health effects / safety hazards: Potassium dichromate is highly dangerous to skin, eyes and mucous membrane. It may cause severe damage by ingestion, causing vertigo, muscle cramps and death. It should be avoiding the contact by allergic people and it is also a mutagenic and a carcinogen. It can intensify fire.
|
Related Links: |
