Potassium Dichromate Formula - Potassium Dichromate Uses, Properties, Structure and Formula
Potassium dichromate, also called potassium dichromate(VI), is a brightly colored and toxic inorganic chemical with many industrial applications.
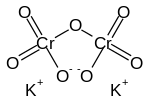
Formula and structure: The chemical formula for potassium dichromate is K2Cr2O7 and its molar mass is 294.185 g/mol. It is an ionic compound with two potassium ions (K+) and the negatively charged dichromate ion (Cr2O7-), in which two hexavalent chromium atoms (with oxidation state +6) are each attached to three oxygen atoms as well as a bridging oxygen atom.
Occurrence: Potassium dichromate occurs naturally in mineral form as lopezite, a very rare mineral.
Preparation: Potassium dichromate is produced industrially by reacting potassium chloride (KCl) with sodium dichromate (Na2Cr2O7). It is also obtained from its related compound, potassium chromate (K2CrO4), which reacts with acids to give the dichromate salt.
Na2Cr2O7 + 2 KCl → K2Cr2O7 + 2 NaCl
Physical properties : It is a bright red-orange crystalline solid with a density of 2.676 g/mL, melting point of 398 °C and boiling point of 500 °C, when it decomposes. It is odorless and highly water soluble.
Chemical properties: Potassium dichromate readily ionizes in water to give chromate (CrO42-) and dichromate (Cr2O72-) ions in equilibrium. It is a moderate oxidizing agent widely used in organic chemistry. It is a stable solid under normal conditions, but it decomposes upon heating to give potassium chromate (K2CrO4) and chromic anhydride (CrO3) with the evolution of oxygen.
4K2Cr2O7 → 4K2CrO4 + 2Cr2O3 + 3O2
It reacts reversibly with bases such as potash (K2CO3) to give a yellow solution of chromate salts.
K2Cr2O7 + K2CO3 → 2 K2CrO4 + CO2
It reacts with cold dilute acids to give chromic anhydride, and with concentrated acids, it gives chromate salts and oxygen.
Uses: Potassium dichromate is used for preparing strong cleaning solutions for glassware and for etching materials. It also has uses in leather tanning, photographic processing, cement, and wood staining. It is used as an oxidizing agent in many applications, and is also used to prepare various products such as waxes, paints, glues, etc.
Health effects/safety hazards: As a hexavalent chromium compound, potassium dichromate is carcinogenic and highly toxic. It is also very corrosive and skin/eye contact can cause severe irritation and burning sensation, and even lead to blindness. It is also known to affect reproductive health and acts as a mutagenic agent (affects the genetic material and harms unborn children).
|
Related Links: |
