Potassium Sulfate Formula
Potassium sulfate, also known as arcanite or sulfate of potash, is an inorganic salt that is mostly used as fertilizer.
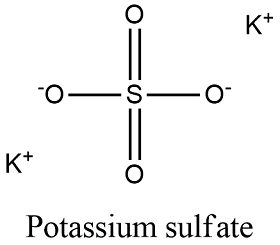
Formula and structure: The potassium sulfate chemical formula is K2SO4 and its molar mass is 174.259 g/mol. It can be found in two crystalline forms, an orthorhombic geometry or a tetrahedral geometry. The molecule is formed by two potassium K+ cations and 1 complex anion sulfate SO42-; the sulfate has a tetrahedrical structure with a central sulfur atom with two oxygen double bond and two oxygen attached through simple bonds and there is one negative net over these two oxygen, which allows to form the ionic bond with the potassium atoms. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium sulfate can be found in nature as a component of minerals as stassfurt salt and kainite, leonite, glaserite and others.
Preparation: Most of the potassium sulfate produced in the world, comes from the reaction of potassium chloride with sulfuric acid.
2 KCl + H2SO4 → 2 HCl + K2SO4
Another method, the Hargreaves process, uses sulfur dioxide, oxygen, water and potassium chloride for producing potassium sulfate.
SO2 + O2 + H2O + 2 KCl → K2SO4 + 2 HCl
Physical properties: Potassium sulfate is a white, odorless solid. Its density is 2.66 g/mL and the melting and boiling point are 1069 ºC and 1686 ºC, respectively. Potassium sulfate is highly soluble in water and the solubility increases with the temperature. However, it is insoluble in acetone and ethanol.
Chemical properties: Potassium sulfate suffers several reactions that allow the use of these compounds for getting multiple compounds in organic synthesis. For example, when the potassium sulfate is acidified by sulfuric acid, the resulting products are potassium bisulfate (KHSO4). A second example is the reduction of the potassium sulfate at high temperatures to form potassium sulfide.
Uses: Potassium sulfate, similar to other sulfates, is used as a fertilizer. It has a high content of potassium and sulfur that are elements required by the plants. Moreover, it is used in the production of glass.
Health effects/safety hazards: Potassium sulfate is slightly irritant for eyes and mucous. It is not flammable.
|
Related Links: |
