Sodium Bisulfite Formula - Sodium Bisulfite Uses, Properties, Structure and Formula
Sodium bisulfite is a common inorganic salt, also called as sodium hydrogen sulfite.
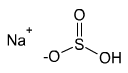
Formula and structure: The chemical formula of sodium bisulfite is NaHSO3. Its molecular formula is HNaO3S and its molar mass (molecular weight) is 104.06 g/mol. It is an ionic compound composed of the sodium cation (Na+) and bisulfite anion (HSO3-). In the bisulfite ion, the central sulfur atom is bonded to a hydroxyl group (OH) and two oxygen atoms through single and double bonds that are in resonance.
Preparation: Sodium bisulfite is prepared industrially by passing sulfur dioxide gas through the aqueous solutions of bases like sodium hydroxide or sodium bicarbonate.
SO2 + NaOH → NaHSO3
Physical properties: Sodium bisulfite is a white solid with a mild sulfurous odor. Its density is 1.48 g/mL and melting point is 150 °C.
Chemical properties: Sodium bisulfite dissociates in water to give the bisulfite and sodium ions. It is a weak acid and attacks metals. It acts as a mild reducing agent and is used to reduce many functional groups in organic synthesis. It decomposes on heating or in the presence of acids to release sulfur dioxide gas.
NaHSO3 + HCl → NaCl + H2O + SO2
It can react violently with strong oxidants and acids, causing fires or explosions.
Uses: Sodium bisulfite is used as a food additive and a food preservative. It is also used in purification and decolorization processes during production of various chemicals. It has applications in wastewater treatment, in drinking water treatment, in prevention of corrosion, wine making, and DNA sequencing. It is also used as a flame retardant, anti-scaling agent and a bleaching agent.
Health effects/safety hazards: Exposure to sodium bisulfite powder or concentrated solutions of sodium bisulfite can be very irritating to the skin, eyes, throat and mucous membranes. It can be very harmful if swallowed, and can cause severe diarrhea or even death. It reacts violently with acids to release the pungent sulfur dioxide gas, which is a severe irritant to eyes and mucous membranes.
|
Related Links: |
