Sodium hydrogen carbonate Formula - Sodium hydrogen carbonate Uses, Properties, Structure and Formula
Sodium hydrogen carbonate is a weakly basic inorganic compound, commonly called as sodium bicarbonate or baking soda.
Formula and structure: The chemical formula of sodium hydrogen carbonate is NaHCO3, and its molar mass is 84.007 g/mol. Its chemical structure is shown below. It is an ionic compound made of sodium cations (Na+) and bicarbonate anions (HCO3-).
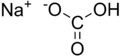
Occurrence: Sodium hydrogen carbonate occurs naturally as the mineral nahcolite, which is found in many mineral springs. This is a major source of this material.
Preparation: Sodium hydrogen carbonate is also prepared on large scales by the Solvay process, in which sodium chloride reacts with ammonia, carbon dioxide and water to give NaHCO3 along with ammonium chloride salt (NH4Cl).
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
Another main preparation method involves dissolving soda ash (sodium carbonate mineral) in water and passing carbon dioxide through the solution.
Na2CO3 + CO2 + H2O → 2 NaHCO3
Physical properties: Sodium hydrogen carbonate is an odorless, white crystalline solid with a density of 2.2 g/mL. It is also commonly available as a fine white powder, with a density of 1.2 g/mL. Its melting point is 50 °C, and it is highly water soluble.
Chemical properties: NaHCO3 dissolves in water to give weakly acidic carbonic acid (H2CO3) and the basic hydroxide ions, making it an overall weak base. Sodium hydrogen carbonate reacts with acids to give salts and carbonic acid, which then decomposes into carbon dioxide gas and water:
NaHCO3 + HCl → NaCl + H2CO3 → H2O + CO2
It decomposes upon heating into sodium carbonate, water and carbon dioxide:
2 NaHCO3 → Na2CO3 + H2O + CO2
It also reacts with bases such as sodium hydroxide to form sodium carbonate:
NaHCO3 + NaOH → Na2CO3 + H2O
Uses: Sodium hydrogen carbonate is commonly used as an antacid, in baking powder, as odor absorber, drying agent, and in toothpastes. It is also used as a fire retardant, in fireworks, for mild disinfecting, pest control, pH balancing, paint removal and metal cleaning applications.
Health effects/safety hazards: It is not considered toxic or hazardous. However, eye or skin contact with the concentrated NaHCO3 solutions can cause severe eye and skin irritation.
|
Related Links: |
