Sodium Nitrate Formula - Sodium Nitrate Uses, Properties, Structure and Formula
Sodium nitrate is an important inorganic salt, which is used as a food additive among many other applications.
Formula and structure: The chemical formula of sodium nitrate is NaNO3. Its molecular formula is NNaO3 and its molar mass is 84.9947 g/mol. It is the sodium salt of nitric acid (HNO3), and hence, is composed of the sodium cation (Na+) and nitrate anion (NO3-). The nitrate ion has the central nitrogen atom bonded to three oxygen atoms, through one double bond and two single bonds, which are in resonance with each other.
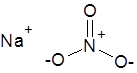
Occurrence: Sodium nitrate occurs naturally as large mineral deposits (called nitratine) in dry regions, especially in the deserts of South America, so it is also called as Chile/Peru saltpeter. The sodium nitrate deposits are found as an impure nitrate rock or gravel. These natural sodium nitrate salt deposits are the main source of this chemical, after mining and purification.
Preparation: Sodium nitrate is also synthesized industrially for some applications, by reacting nitric acid with a base such as sodium carbonate, sodium bicarbonate or sodium hydroxide.
HNO3 + NaOH → NaNO3 + H2O
It is also prepared by reacting ammonium nitrate with bases, as this reaction is less exothermic (heat producing) than the strong acid-strong base reaction above.
NH4NO3 + NaOH → NaNO3 + NH4OH
Physical properties : Sodium nitrate is a white crystalline solid with a density of 2.26 g/mL and a melting point of 308 °C. It exists as either trigonal or rhombohedral crystals.
Chemical properties: Sodium nitrate is the salt of a strong acid and hence, dissociates completely in water into sodium and nitrate ions. It is a stable solid at room temperature, however upon prolonged heating, it can explode and release toxic fumes. It is a strong oxidizer and reacts violently with strong reducing agents and flammable materials.
Uses: Sodium nitrate is used as a food preservative and a salt substitute. It is also used in dental products, explosives, smoke bombs, fertilizers, wastewater treatment, energy storage materials, and solid rocket propellants.
Health effects/safety hazards: Sodium nitrate is a strong oxidizer and can cause skin and eye irritation on exposure. Swallowing high concentrations of sodium nitrate can injure the gastrointestinal tract, and cause severe abdominal pain, diarrhea and nausea.
|
Related Links: |
