Sodium Sulfide Formula
Sodium sulfide, also known as disodium monosulfide, is an inorganic compound used in the paper industry, treatment of water and textile industry.
Formula and structure: The sodium sulfide formula is Na2S the molar mass is 78.0452 g mol-1. The salt also has a hydrated form 240.18 g mol-1 The molecule is formed by two sodium cations Na+ and one sulfur anion -2. The geometry of the structure is a antifluorite, with Na+ centres are eight-coordinate, being centered in a "box" for eight S−2 centers. Its chemical structure can be written as below, in the common representations used for organic molecules.
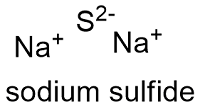
Occurrence: Sodium sulfide can be found in nature in hot springs, meteorites, volcanoes.
Preparation: Sodium sulfide is produced through the reduction of sodium sulfate using coa above 950 ºCl:
Na2SO4 + 2 C → Na2S + 2 CO2
A second method is the reaction between sodium carbonate and barium sulfide.
Physical properties: Sodium sulfide is colorless, hygroscopic and rotten egg like odor soli. Its density is 1.856 g mL-1 or 1.58 g mL-1 in its pentahydrate form. Sodium sulfide melting point is 1176 °C and it is partially soluble in water. It is slightly soluble in ethanol and insoluble in ether.
Chemical properties: Sodium sulfide can suffer a protonation in water, thus the sodium can be replaced by a proton according to the reaction:
S2− + H+ → SH−
SH has a basic character, which allows the protonation in aqueous solutions to form:
SH− + H2O → SH2 + OH-
Uses: Sodium sulfide can be used in the pulp and paper production and it is also used in the water treatment. It is also to precipitate some metals and can be used as a blenching agent in textiles and also in paper production.
Health effects / safety hazards: Sodium sulfide is alkaline and can cause severe skin and eyes damage. It can easily react with acids to produce the highly toxic hydrogen sulfide.
|
Related Links: |
