Stannous Oxide Formula
Stannous oxide, also known as tin (II) oxide or tin monoxide, is an inorganic compound used as raw material and precursor in chemical industry.
Formula and structure: The stannous oxide chemical formula is SnO and its molar mass is 134.709 g mol-1. The molecule is formed by a tin cation I+2 and an oxygen anion O2-. Its lattice structure is orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
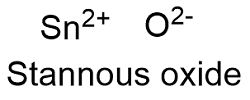
Occurrence: Stannous oxide is found in nature in some minerals such as romarchite. It is extremely rare.
Preparation: Stannous oxide can be prepared from the stannous (II) oxalate through a reaction that is preformed under a carbon dioxide atmosphere:
SnC2O4· 2H2O → SnO + CO2 + CO + 2 H2O
Physical properties: Stannous oxide is a brown to black or blue crystalline solid. Its density is 6.45 g mL-1. Stannous oxide melting point is 1080 ºC and above this temperature decomposes. It is insoluble in water and can react with acids and basic solutions.
Chemical properties: Stannous oxide is an amphoteric oxide, thus it can react with acid and basic solutions. Moreover, stannous oxide is a reducing agent due it can be easily oxidized from Sn (II) to Sn (IV). This reaction can also be made with air:
2 SnO + O2 → 2 SnO2
Stannous oxide is also used to produced other Sn compounds such as Sn3O4, which are produced due it is easily oxidized:
4 SnO → Sn3O4 + Sn
Sn3O4 → 2SnO2 + Sn
Uses: Stannous oxide is used as catalyst and also to produce other tin compounds such as tin (IV) oxide or stannous salts. Moreover, it is used in the productions of glass and ceramic and can also be a catalyst in many organometallic reactions.
Health effects / safety hazards: Stannous oxide is dangerous by inhalation or ingestion. It can cause serious eyes and mucous damage and skin irritation. It is not flammable.
|
Related Links: |
