Strontium chloride Formula
Strontium chloride, also known as strontium dichloride, is an inorganic salt known to be a raw material to obtain other strontium compounds. It is also use to produce fireworks and dental care products.
Formula and structure: The strontium chloride chemical formula is SrCl2 and it has two forms: the anhydrous and the hydrated form. The anhydrous molar mass is 158.53 g mol-1, while 266.62 g mol-1 is the molar mass of the hexahydrate form. In general, the molecule is formed by a strontium cation Sr+2 and two chloride anion Cl-1 which form an structure octahedral with six anions surrounded a cation. Its chemical structure can be written as below, in the common representations used for organic molecules.
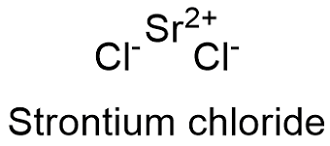
Occurrence: Strontium chloride is not produce in nature, it is jus obtained from chemical methods.
Preparation: Strontium chloride is produced by the same chemical process that is used to produce strontium fluoride SrF2. Strontium chloride is prepared by the reaction between hydrochloric acid and strontium hydroxide or strontium bicarbonate or carbonate:
Sr(OH)2 + 2 HCl → SrCl2 + 2 H2O
SrCO3 + 2 HCl → SrCl2 + H2O + CO2
Physical properties: Strontium chloride is a colorless to white crystalline solid or powder. Its density is 3.052 g mL-1 (anhydrous) and 2.672 g mL-1 (hexahydrate). The melting points are 874 ºC (anhydrous) and 61 ºC (hexahydrate), while the boiling point for the anhydrous salt is 1250 ºC. Strontium chloride is soluble in water, poorly soluble in methanol, ethanol and acetone and insoluble in ammonia.
Chemical properties: Strontium chloride has the same chemical properties that its homologous salt strontium fluoride, thus it has a very polarized bound Sr-Cl due to the electronegativity of chloride atom. However, the polarization is not lower than in the case of a bound with the fluoride atom but it is still enough to promote a change of the structure when the strontium chloride is in the gaseous state and bring a high electroconductivity.
Uses: Strontium chloride has been used for many years as a source of strontium or like a raw material to produce other strontium compounds; for example, the strontium chromate which is produced from sodium chromate and strontium chloride:
SrCl2 + Na2CrO4 → SrCrO4 + 2 NaCl
It has been also use to manufacture firework due to the cation strontium produce a red color when in contact with a flame. A large quantity if strontium chloride is also used in the production of toothpaste for tooth sensitivity.
Health effects / safety hazards: Strontium chloride can irritate skin, eyes and mucous. It is not poisonous like strontium fluoride. It is not flammable and it does not react with other chemical species.
|
Related Links: |
