Titanium oxide Formula
Titanium oxide, also known as titanium monoxide, is the second most common oxide of titanium. It is used mainly in the manufacturing of electronic devices due its superconductivity.
Formula and structure: The titanium oxide chemical formula is TiO and its molecular weight is 63.886 g mol-1. Titanium oxide is a non-stoichiometric compound, so that their elemental composition cannot be represent by an integer number. Consequently, the most correct representation for titanium oxide would be TiOx, where 1.3>x>0.82. Then, the structure of titanium oxide has vacancies of cation Ti or anion O: it has been determinated that 15% of the ions lattice sites are not occupied. At high temperatures, the titanium oxide structure has a cubic geometry. Its chemical structure can be written as below, in the common representations used for organic molecules.
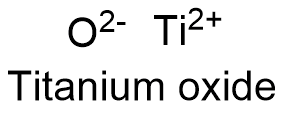
Occurrence: Titanium monoxide, similar to other titanium oxides as TiO2 can be found in some minerals such as ilmenite. Interesting, some scientific studies had determinated titanium oxide is one of the compound present in the interstellar medium.
Preparation: Titanium oxide is prepared by heating titanium dioxide and titanium metal at 1500. Since TiO is not largely used in industries, its preparation is not made in large-scale.
Physical properties: Titanium oxide has the physical appearance of bronze crystalline solid. Its melting and boiling point are 1750 ºC and >3000 ºC. Its density is 4.95 g mL-1. Titanium oxide is not soluble in water, but similar to other oxides, it can react with acid solutions.
Chemical properties: The titanium oxide is not largely use when it is compared with the titanium dioxide, which is very important for chemical and pharmaceutical industries. Instead, titanium oxide has found application in material science due its superconductivity associated with its non-stoichiometrical character. As described above, the most adequate formula for titanium oxide would be TiOx; the variation of x is directly influenced by the temperature, so that the conductive of TiO also will change.
Uses: Titanium oxide is used in material and electronic fields. It is used as component of ceramic, glass and optic components. It has found application as component in electrochemical cells due its superconductivity.
It is also used as a pigment purple.
Health effects/safety hazards: Titanium oxide can irritate eyes and it is also a respirator system irritation. It can react with acids. It is not flammable.
|
Related Links: |
Related Topics
Titanium Facts
