Tungsten (IV) carbide Formula
Tungsten (IV) carbide, also known as tungsten tetracarbide or just tungsten carbide, is a chemical compound used to produce industrial equipments, jewerly and cutting utensils.
Formula and structure: The tungsten carbide chemical formula is WC and its molar mass is 195.85 g mol-1. The molecule is formed by the tungsten IV cation W+4 and the carbon (or carbide) anion C-4. The carbide is a type of chemical compound where the carbon is bounded to a lesser electronegative element such a W, in this case the atoms form a triple bond. The structure is hexagonal or cubic depending on the temperature. Its chemical structure can be written as below, in the common representations used for organic molecules.
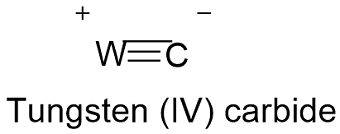
Occurrence: Even when many compounds of tungsten are found in nature, tungsten carbide is only obtained by chemical synthesis.
Preparation: Tungsten carbide is mainly obtained through the reaction between the tungsten metallic and carbon at high temperatures (1500-2000 ºC). However, there are other methods such as the reaction between tungsten hexafluoride and methane in the presence of hydrogen (reaction 1); in this reaction the methane can also be replaced by methanol.
WCl6 + H2 + CH4 → WC + 6 HCl
WF6 + 2H2 + CH3OH → WC + 6 HF + H2O
Physical properties: Tungsten carbide is a grey to black lustrous solid and its density is 15.6 g mL-1. Tungsten carbide melting and boiling points are 2830 ºC and 6000 ºC, respectively. It is insoluble in water, but is soluble in nitric acid and hydrofluoric acid. Tugnsten carbide has hardness extremely elevated, 9 on the Mohs scale.
Chemical properties: Tungsten carbide has very interesting properties, first it shows an elevated hardness, just surpassed by diamond and corundum. Thus, the tungsten carbide is highly demanded in the industry of cutting tools. It also has a high electrical resistivity, which is required to electrical applications and it is one of the compounds which exhibits the higher boiling and melting point.
Uses: The tungsten carbide represented the 60 % of the total of tungsten compounds consumed on the Earth. This is mainly used to manufacture cutting materials and tools because it is very resistance to abrasion, corrosion and high temperature; for example: surgical instruments: scissors, forceps and hemostats. On the military industry it is used to produce ammunition and mining tools. Moreover, many countries use the tungsten carbide to promote nuclear chain reaction for nuclear weapons. Nowadays, tungsten carbide is used in the jewelry manufacture too.
Health effects / safety hazards: Tungsten carbide is suspected to be a carcinogen agent and it is also an irritator for eyes and mucous. Moreover, tungsten carbide is also flammable.
|
Related Links: |
