Vinegar Chemical Formula
Vinegar is a solution of acetic acid, the concentration of it, is approximately 5-10%, resulting in a diluted acidic solution that is used in food industry, household chores and as a disinfection agent.
Formula and structure: The chemical structure of the vinegar is CH3COOH and its molecular weight is 60.05 g/mol. It is a solution of the second simplest carboxylic acid with only two carbon atoms in its skeleton. It is formed by one methyl group (-CH3) that has the a sp3 or tetrahedral conformation and one carboxylic carbon (-C=O) with has a conformation sp2 or planar. Due to the solvation by water molecules, the correct representation of vinegar is an equilibrium of CH3COOH and the ions CH3COO- + H+, where water is responsible for the dissociation of H+ from the acid. Its chemical structure can be written as below, in the common representations used for organic molecules.
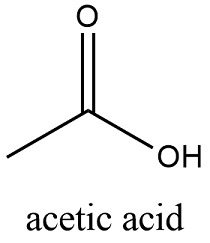
Occurrence: Vinegar is produced by bacteria and yeast during the fermentation of sugars, especially the yeast Saccharomyces cerevisiae is highly used for preparing these fermentations.
Preparation: Vinegar is prepared from methanol carboxylation, in a reaction in a reaction between the methanol and carbon monoxide.
CH3OH + CO2 → CH3COOH + 1/2 O2
One part of the world production of vinegar comes from bacterial fermentation, especially for preparing vinegar, it is used the vinegar produced by microbes in order to get a higher purity for human consume.
Physical properties: Vinegar is a colorless liquid with corrosive pungent vinegar-like odor with a sour taste. Its density is 1.05 g/mL, and its melting and boiling point are 16 ºC and 118 °C, respectively. It is highly miscible in water, methanol and ethanol.
Chemical properties: Vinegar is a weak organic acid, which means that it is not completely dissociated in water when a solution of this compound is prepared and its reaction should be represented as a reversible reaction:
CH3CO2H ⇌ CH3CO2− + H+
The pH of solution of vinegar is not too low and it oscillated between 5-10%.
Its highly miscibility in water is due to the formation of hydrogen bonds between the oxygen atoms and the hydrogen atoms of the water.
Uses: Vinegar is largely used in the food industry as a flavoring agent and preservative and in household, where it is used in food and for cleaning or disinfection. It is also very used to prepare buffer solutions along with sodium acetate for regulating the pH of solutions.
Health effects/safety hazards: when concentrate, vinegar is very corrosive to eyes, skin and mucous membranes upon inhalation or contact. It can cause severe damage on the eyes and it is flammable over temperatures of 40 ºC.
|
Related Links: |
