Zinc oxide Formula
Zinc oxide is an inorganic chemical compound used as ingredient in medicine preparation or over-the-counter drug. It is also largely used as additive in pigments and semiconductor in industries.
Formula and structure: the zinc oxide chemical formula is ZnO. Its molar mass is 81.379 g mol-1. The molecule is formed by the cation Zn+2 and the anion O-2. Zinc oxide has two possible structures: hexagonal and cubic, but hexagonal crystals are most common. Its chemical structure can be written as below, in the common representations used for organic molecules.
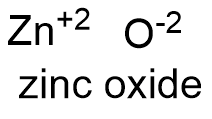
Occurrence: Zinc oxide is found in nature in zincite, a mineral manly found in New Jersey, US. Zincite also has a hexagonal crystal structure.
Preparation: There are several processes to synthesize zinc oxide. The main routes are the French and the American process. In French process, metallic zinc is vaporized and the vapor are oxidized with preheated air. The American process use as raw compounds different zinc composites, which are reduced by coal, producing zinc vapors. Then, the zinc vapors are oxidized by oxygen present in the air, similar to the French process.
Other way to synthesize zinc oxide is the wet process, which consists in purify zinc sulfate or chloride through the precipitation with carbonate. Then, the precipitate is calcined to obtain zinc oxide.
Physical properties: Zinc oxide is a gray to white, odorless powder or a granular solid. Its melting point is 1975 °C and its density is 5.68 g mL-1. It is not soluble in water, however it is soluble in acid and alkaline solutions.
Chemical properties: ZnO is an amphoteric oxide that can be dissolved in acids or alkali through the reactions:
ZnO + 2 H+ → Zn+2 + H2O
ZnO + 2 OH- → Zn+2 + H2O
Uses: Zinc oxide is largely use in pharmaceutical and chemical industry. In pharmaceutical industry, it is used as photosensitive agents to protect the skin from the sun burn and as an astringent and antibacterial component. ZnO is sold in its pure form as a dermatological agent. In chemical industry, it is used as an additive in the manufacture of rubbers, pigments, lubricants and ceramic. Moreover, ZnO is used as a semiconductor in electronics' manufacturing.
Health effects/safety hazards: In spite of zinc oxide's use as a drug, it is very toxic to the environment, especially to aquatic organisms. It is not dangerous to human in concentration lesser to 25%. Zinc oxide is not flammable and it does not show incompatibility with other chemical compounds but when it is heated, emits toxic fumes.
|
Related Links: |
Related Topics
Zinc Sulfide Formula
Ionic and Metallic Bonding
Combustion Reaction Examples
