Enduring Understanding 6.A: Reversible Reactions
- Chemical reactions are reversible at a molecular level - they can proceed in either direction.
- When a reaction and its reverse are happening at the same rate, such that the concentration of reactants and products are not changing over time, this is called dynamic chemical equilibrium.
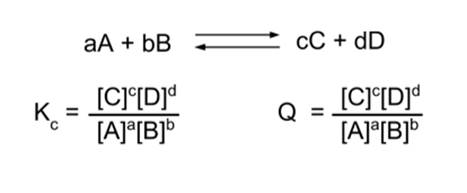
- Chemical equilibrium can be expressed mathematically:
- When the reaction is in equilibrium, the concentrations of products and reactants can be used to calculate the equilibrium constant, Kc. The c denotes concentration; an equilibrium constant that uses pressure is Kp.
- Example : The reaction:
- At equilibrium in a rigid container, the concentration of SO2 and Cl2 is 0.085 mol/L and the concentration of SO2Cl2 is 0.915 mol/L. The Kc can be calculated:
- The reaction quotient is similar, but for situations in which the reaction mixture is NOT at equilibrium.
- Example : If 0.50 moles of SO2Cl2 and 0.20 moles of SO2 and Cl2 are combined in a 1L container, Q can be calculated:
- Q and K can be compared, to determine which direction a chemical reaction will go in.
- If K > Q, the reaction will proceed in the forward direction.
- If K < Q, the reaction will proceed in the reverse direction.
- When K = Q, the reaction is at equilibrium. The rate of the forward and reverse reactions are equal.
- Note that only reactants that can change concentration or pressure contribute to the calculation of a K or a Q.
- Solid and liquid reagents are not included in equilibrium constant calculations.
- Example: The reaction quotient for the reaction:
- Is given by:
- The solid reagent ClO, cannot change in concentration, so it is not included in the equation.
- Similarly, the Kp for the reaction P4 (g) + 6F2 (g) ⇆ 4PF3 (l) is given by 1/[PP4] [PF2]6. The liquid product does not have a pressure, so cannot contribute to the pressure equilibrium constant.
|
Related Links: Chemistry Chemistry Quizzes AP Chemistry Notes Gibbs Free Energy |
To link to this Reversible Reactions page, copy the following code to your site:
