Ammonia Formula - Ammonia Uses, Properties, Structure and Formula
Ammonia, also called azane or nitrogen trihydride, is the simplest inorganic base and an important source of nitrogen for many applications.
Formula and structure: The chemical formula of ammonia is NH3, and its molar mass is 17.03 g/mol.
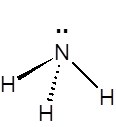
The ammonia molecule has a trigonal pyramidal shape, with nitrogen connected to the three hydrogen atoms. The nitrogen atom has a lone electron pair, which makes ammonia a base. NH3 is a polar molecule which readily forms hydrogen bonds, making it highly miscible with water.
Occurrence: Ammonia occurs naturally in the body, and is secreted by the kidneys to neutralize excess acid. It is also found in small amounts in rainwater, volcanic areas, and even the atmosphere.
Preparation: Ammonia is produced commercially by the Haber-Bosch process, in which elemental hydrogen and elemental nitrogen are reacted in the presence of a metal catalyst to give ammonia gas. The reaction is performed at high pressures and high temperature (400–550 °C).
N2 + 3H2 → 2NH3
Physical properties: Ammonia is a colorless gas with a sharp, penetrating odor. Its boiling point is -33.35 °C, and its freezing point is -77.7 °C. NH3 gas can be liquefied, however, due to its extremely low boiling point, liquid ammonia must be stored at low temperature and high pressure.
Chemical properties: Ammonia is a weak base. It combines with various acids to form ammonium salts, which are important chemicals in many industries. Ammonia readily dissolves in water in an exothermic reaction, to form aqueous ammonia solution, also called as ammonium hydroxide (NH4OH).
NH3 + H2O → NH4OH
Liquid ammonia is an important non-aqueous solvent, which can dissolve many alkali and alkaline-earth metals to produce blue-colored conductive solutions.
Uses: Ammonia is the precursor to various important nitrogen compounds, such as urea, amino acids, phenol, acrylonitrile, hydrogen cyanide, soda ash, nitric acid, and many others. It is also used for the production of fertilizers, polymers, synthetic fibers (nylon, rayon), explosives (TNT, nitroglycerin), cleaning agents, and refrigerants.
Health hazards/ health effects: Although it is widely available in nature and used for many household purposes, ammonia is considered toxic by inhalation. NH3 fumes have a sharp and pungent odor which can seriously irritate the eyes, nose, mucous membranes and skin, and damage the respiratory tract. At high concentrations, exposure to ammonia gas can cause permanent lung damage and death.
|
Related Links: |
