Sodium Thiosulfate Formula - Sodium Thiosulfate Uses, Properties, Structure and Formula
Sodium thiosulfate is an important inorganic salt with several medical uses. It is also called sodium hyposulfite or 'hypo'.
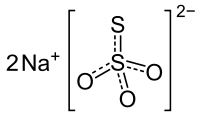
Formula and structure: The chemical formula of sodium thiosulfate is Na2S2O3 and its molar mass is 158.11 g/mol. It is also available as its pentahydrate salt (Na2S2O3.5H2O), with a molar mass of 248.18 g/mol. It is an ionic compound composed of two sodium cations (Na+) and the negatively charged thiosulfate anion (S2O3-), in which the central sulfur atom is bonded to three oxygen atoms and another sulfur atom, all through single and double bonds with resonance character. The solid exists in a monoclinic crystal structure.
Preparation: Sodium thiosulfate can be prepared by heating sulfur with either aqueous sodium sulfite solution or aqueous sodium hydroxide solution.
6 NaOH + 4 S → Na2S2O3 + 2 Na2S + 3 H2O
Physical properties : Sodium thiosulfate is a white crystalline solid which is odorless and highly water soluble. It has a density of 1.667 g/mL and a melting point of 48.3°C.
Chemical properties: Sodium thiosulfate is a neutral salt which readily dissociates in water to give sodium and thiosulfate ions. Na2S2O3 is a stable solid under normal conditions, but decomposes upon heating to give sodium sulfate and sodium polysulfide:
4 Na2S2O3 → 3 Na2SO4 + Na2S5
It also decomposes when treated with dilute acids to give sulfur and sulfur dioxide (called 'clock reaction'):
Na2S2O3 + 2 HCl → 2 NaCl + S + SO2 + H2O
It reacts stoichiometrically (in equimolar amounts) with aqueous solutions of iodine, and so, it is widely used in laboratories for iodine based titrations.
Uses: Sodium thiosulfate is used in several pharmaceutical preparations and also has various medical properties. It is an important antidote used for treating cyanide poisoning. Apart from its medical uses, it also has applications in water treatment, neutralizing bleach, leather tanning, gold extraction, photographic processing, and chemical heating pads.
Health effects/safety hazards: Sodium thiosulfate is not a toxic material and is used for medical purposes. However, when it decomposes, it produces toxic sulfur oxide fumes, which can cause irritation to eyes, skin and mucous membranes.
|
Related Links: |
