Intermolecular Forces
Forces of attraction that draw molecules together are called intermolecular forces. The three main intermolecular forces are London dispersion forces, dipole-dipole interactions and hydrogen bonding.
1. London dispersion forces are caused by the motion of electrons. As the electrons spin around the nucleus of an atom, the number of electrons on one side of the atom could be greater than the number on the other side. This creates an instantaneous dipole. The uneven distribution of charge on the atom will induce the same dipole on a nearby atom, causing an attraction.
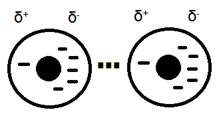
2. Dipole-dipole interactions occur when two polar molecules attract to one another. These are permanent dipoles meaning they are different from the instantaneous dipoles created in London dispersion forces. The polar molecules are caused by the uneven sharing of electrons in a bond due to the difference in electronegativity of the atoms in the molecule. The atom with the larger electronegativity will pull the electron in the bond closer to it, creating a negative and positive area in the molecule.

3. Hydrogen bonds are dipole-dipole interactions that are stronger due to the presence of hydrogen attached to a highly electronegative atom. The hydrogen atom is the smallest reactive element which allows the polar molecules to become very close to one another. This creates a much stronger attraction between the molecules.
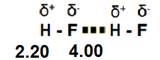
1. London dispersion forces are caused by the motion of electrons. As the electrons spin around the nucleus of an atom, the number of electrons on one side of the atom could be greater than the number on the other side. This creates an instantaneous dipole. The uneven distribution of charge on the atom will induce the same dipole on a nearby atom, causing an attraction.

2. Dipole-dipole interactions occur when two polar molecules attract to one another. These are permanent dipoles meaning they are different from the instantaneous dipoles created in London dispersion forces. The polar molecules are caused by the uneven sharing of electrons in a bond due to the difference in electronegativity of the atoms in the molecule. The atom with the larger electronegativity will pull the electron in the bond closer to it, creating a negative and positive area in the molecule.

3. Hydrogen bonds are dipole-dipole interactions that are stronger due to the presence of hydrogen attached to a highly electronegative atom. The hydrogen atom is the smallest reactive element which allows the polar molecules to become very close to one another. This creates a much stronger attraction between the molecules.
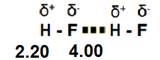
|
Related Links: Chemistry Organic Chemistry Mass Spectroscopy Physical Properties of Alkanes |
To link to this Intermolecular Forces page, copy the following code to your site:
