Allyl chloride Formula
Allyl chloride, known by the IUPAC name 3-chloropro-1-ene, is a very useful compound in organic chemistry used as precursor to produce polymers and plastic.
Formula and structure: The allyl chloride chemical formula is C3H5Cl and its molar mass is 76.52 g mol-1. The extended formula is CH2=CHCH2Cl. The molecule shows a typical structure of allyl compounds (CH2=CHCH2R, which is formed by a methyl group –CH3 joined to a vinyl group –CH=CH2) but the group R is a chlorine atom. The geometry is mostly planar to allow the resonance between the pi electrons through the sp2 orbital. Its chemical structure can be written as below, in the common representations used for organic molecules.
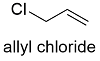
Occurrence: Allyl chloride is not found in nature, it is synthesized by chemical processes.
Preparation: Allyl choride can be prepared by the chlorination of propene at high temperatures.
CH2=CHCH3 + Cl2 → CH2=CHCH2Cl
It can also be produced by the chlorination of propylene or by the reaction of allylalcohol and phosphorous chloride.
Physical properties: Allyl chloride is a colorless, yellow or brown liquid with a pungent odor.. Its density is 0.94 g mL1- and its melting point is -135 ºC and its boiling point is 45 ºC. It is not soluble with water, but it is soluble in ether, acetone, benzene and chloroform.
Chemical properties: Allyl chloride chemical properties are given by the allyl group. This grouo is widely studied and used in synthesis of organic compounds. The allyl group is stabilized by resonance between the three atoms which form the system, thus the three carbon atoms have sp2 molecular orbital for the delocalization of electrons.
Uses: Allyl chloride is largely used as precursor to produce epichlorohydrin, a compound used on the production of some plastic. It can also be used to produce allyl alcohol or allylsilane, allylamines, all of them very useful in the chemical industry. It is an alkylating agent used by the pharmaceutical and chemical industries.
Health effects / safety hazards: Allyl chloride is a possible carcinogen, thus the long term contact must be avoided. It is an irritator to eyes, skin and respiratory tract. It is toxic by ingestion. Moreover, the compound is flammable.
|
Related Links: |
