Aluminum Nitrate Formula
Aluminum nitrate, also known as aluminium (III) nitrate or Nitric Aluminum salt, is a salt largely used in industry to produce alumina.
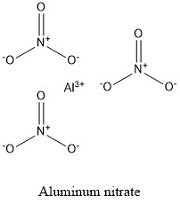
Formula and structure: Aluminum nitrate chemical formula is Al(NO3)3 and the molar mass is 212.99 g mol-1. In general it is found as a hydrate salt surrounded by 9 water molecules (nonahydrate form). The nonahydrate chemical formula is Al(NO3)3·9H2O and the molar mass is 375.13 g mol-1. Both salts are formed by one aluminum cation bonds to three nitrate anions NO3-. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Aluminum nitrate is not found in nature.
Preparation: Aluminum nitrate is synthesized from the reaction of nitric acid and aluminum (III) chloride:
AlCl3 + HNO3 → Al(NO3)3 + HCl
It can also be synthesized by the reaction of nitric acid and aluminum hydroxide. Alternatively, aluminum nitrate is prepared through the reaction of aluminum sulfate and barium nitrate:
Al2(SO4)3 + 3Ba(NO3)2 → 2Al(NO3)3 + 3BaSO4
Physical properties: Aluminum nitrate can be found in the anhydrous form as the white odorless hygroscopic powder or the hydrate (nonahydrate) that is a deliquescent solid. The density of the nonahydrate is 1.72 g mL-1. The melting points are 66 ºC (anhydrous) and 74 ºC (nonahydrate). The boiling point is 150 ºC for the anhydrous, while the nonahydrate decomposes. Both forms are highly soluble in water.
Chemical properties: Aluminum nitrate is used as a nitrating agent due to this salt can react with some hydroxides or other salts to form a new nitrate, for example:
Al(NO3)3 + 3 NaOH → Al(OH)3 + 3 NaNO3
Uses: Aluminum nitrate is used as a nitrating agent. It is also used as a precursor in the production of alumina. This salt is also used in some industrial processes as the extraction of the heavy elements as uranium.
Health effects / safety hazards: Aluminum nitrate is a strong oxidizer used, so that it can react in contact with other material causing fire. It is extremely dangerous if inhaled or swallowed and can cause severe irritation to skin, eyes and respiratory tract.
|
Related Links: |
