Amino Acid Formula
Amino acids, also known as α- amino carboxylic acids, are organic compound that are the basis of the proteins.
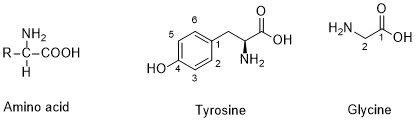
Formula: For a compound being considered amino acid must contain two functional groups: an amino group (-NH2) and a carboxylic acid group (-COOH). There are 22 proteinogenic amino acids in which we can find: alanine, tyrosine, aspartic acid, etc. On the other hand, there are around 500 non-proteinogenic. The molar mass of amino acids varied according to the structure. In the case of glycine, which is the simplest amino acid the molar mass is 75.7 g/mol. Larger amino acids such as tyrosine has a molar mass of 181.09 g/mol. The structure of the amino acids is formed by a central carbon atom (called alpha carbon), to which both groups (NH2 and COOH) are bound. The fourth bond of the carbon atom is complete by one hydrogen or a R alkyl group such as methyl. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Amino acids proteinogenic can be found in all the organisms, from the simplest prokaryotes to the human. The non-proteinogenic amino acids are also found in nature.
Preparation: Amino acids can also be prepared in laboratory using some routes as the Strecker amino acid routes. It can also be produced by the action of genetically modified organisms.
Physical Properties: The physical properties vary according to the specific amino acid to be considered. Most of the amino acids are solids and their solubility in water depends on the structure of each one. They have melting point of around 200 °C.
Chemical Properties: The amino acids can be studied according to some chemical properties as the Isoelectric point, this point establishes the pH in which all the charges in the amino acid molecules are equal to zero. Another important characteristic of amino acids is that all the proprieties regarding solubility and stability depends on the structure of the molecule. For example, if the r side alkyl chain is long, the amino acids will be less soluble in water. A R formed by aromatic group will have a particular absorbance energy, etc.
Uses: Amino acids are used in nature to make the proteins. The proteins are formed through the peptide's bonds of different amino acids until complete long chains. Enzymes, that are a type of protein, can be former for hundred of amino acids. They can also be used as precursors in organic synthesis.
Safety: Depends on the amino acid, it can be harmful for one or other species, while it can be necessary for another one. In general, amino acids are not flammable and do not react violently with other chemical compounds.
|
Related Links: |
