Ammonium Chloride Formula
Ammonium chloride, also known as salmiac or sal ammoniac, is a inorganic salt found in the urine. It has a very important biochemical function maintaining the pH.
Formula and structure: The ammonium chloride chemical formula is NH4Cl and its molar mass is 53.490 g mol-1. The molecule is formed by 2 potassium cations K1+ and 1 dichromate anion Cr2O72-. It is found in both forms: the anhydrous and the three hydrate. Its lattice structure is triclinic. Its chemical structure can be written as below, in the common representations used for organic molecules.
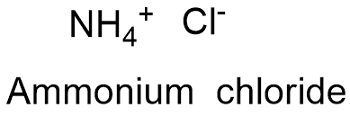
Occurrence: Ammonium chloride is found in mineralogical formations and in this form is called as salt ammoniac. It has also been found in some volcanic vents and ashes.
Preparation: Ammonium chloride can also be obtained by various methodologies. The first one is through the neutralization with HCl of the ammoniacal residue formed in the distillation of coal:
NH3 + HCl → NH4Cl
The second strategy is through the Solvay process to obtain sodium carbonate (or bicarbonate) and where the ammonium chloride is produce as by-product:
NH3 + CO2 + NaCl + H2O → NH4Cl + NaHCO3
In Solvay process the sodium bicarbonate is recovery by filtration and then the ammonium chloride that remains in solution crystallize.
Physical properties: Ammonium chloride is a white, odorless solid. Its density is 1.5274 g mL-1 and the melting and boiling points are 338 ºC and 520 ºC. Ammonium chloride is soluble in water, ethanol, methanol and glycerol and slightly soluble in acetone. It is insoluble in organic solvents as ethyl acetate.
Chemical properties: Ammonium chloride may suffer different reactions of the great value to the chemical and pharmaceutical industries. It can decompose when heated into hydrochloric acid and ammonia (reaction I). Additional, it can react with bases like sodium or potassium hydroxide to also produce ammonia gas. Other important reaction where is used ammonia chloride is the decomposition of carbonates and bicarbonates, forming a salt and ammonia (reaction III).
NH4Cl → NH3 + HCl (I)
NH4Cl + NaOH → NH3 + NaCl + H2O (II)
2 NH4Cl + Na2CO3 → 2 NaCl + CO2 + H2O + 2 NH3 (III)
Uses: Ammonium chloride has a large nitrogen source, so that and similarly to urea it is used as fertilizers and it is added to bacteria and yeast growth media. It is also used to preparing some metals before galvanization that needs a surface free of metal oxides. Moreover, ammonium chloride is used by the pharmaceutical industry to produce cough syrop and expectorants and it is also included in the treatments of metabolic alkalosis and to maintain the urine pH.
Health effects / safety hazards: Ammonium chloride is extremely poisonous and toxic and cause damage to organs by ingestion or long term exposition. It also causes serious eyes damage. It is not flammable and does not react with other chemical substances.
|
Related Links: |
