Ammonium oxalate Formula
Ammonium oxalate, also known as diammonium ethanedioate is the salt of oxalic acid with ammonium. It is a common salt used in analysis to determine metal concentrations.
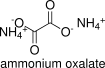
Formula and structure: The chemical formula of ammonium oxalate is (NH4)2C2O4 or NH4OOCCOONH4. Its molecular formula is C2H8N2O4. And its molar mass is 124.096 g mol-1. Oxalate (C2O4)-2 is formed from oxalic acid through the dissociation two acidic hydrogens to form an anion. Two ammonium (NH4)+ are in the molecule as cations: one ammonium to substitute each hydrogen lost. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ammonium oxalate is a constituent of some types of kidney stone. It is found in "guano", which is the accumulation of seabirds, seals or/and bat. It mixture is nitrogen-rich, particularly because the high concentration of ammonium oxalate and urate, consequently can be used as fertilizer and fungicide for plants.
Preparation: Ammonium oxalate can be prepared from a neutralization of a oxalic acid in water. Ammonium carbonate or ammonium hydroxide can be used as ammonium source. In the first reaction, CO2 is produces secondary product.
(NH4)2CO3 + H2C2O4 → (NH4)2C2O4 + CO2 + H2O
2NH4OH + H2C2O4 → (NH4)2C2O4 + 2H2O
Physical properties: Ammonium oxalate is a colorless crystalline powder. It is non-volatile and odorless salt. Its melting point is 131-135 ºC. It is moderately soluble in water and its density is 1.50 g mL-1.
Chemical properties: In solution where the oxalate anion is dissociated, the negative charge is delocalized by the 4 oxygen atoms of ion, forming a very stable chemical specie. Reaching this stable conformation requires all the oxalate atoms have sp2 orbitals. Interestingly, dissociation of oxalic acid forms the oxalate dianion (each charge under carboxylic oxygen); however it can be possible to lose only one proton resulting in the anion hydrogen oxalate (HC2O4-), which can form monobasic oxalate salts similar to oxalate salts.
Uses: Ammonium oxalate is used in blood test, where is added to avoid the coagulation of plasma. Consequently, with the oxalate to form complex with some metals, ammonium oxalate is used to quantify calcium and lead ions concentration in blood and other samples. It is very used in industry to manufacture explosive and polishing substances. Similarly to sodium acetate, ammonium oxalate is also a buffering reagent.
Health effects/safety hazards: Ammonium oxalate can cause skin irritation and corrosion. In eyes, it can cause serious damage. This salt is also combustible and when heated, produces toxic and corrosive fumes including ammonia and nitrogen oxides.
|
Related Links: |
Related Topics
Ionic and Net Ionic Equations
