Aspirin Formula
Aspirin, which the chemical name acetylsalicylic acid (ASA), is medication extensively used around the world as an anti-inflammatory and anti-pyretic.
Formula and structure: The acetylsalicylic acid chemical formula is C9H8O4 and the extended formula is CH3COOC6H4COOH. The molecular mass is 180.159 g mol-1. The molecule is formed by an aromatic ring which has two functional groups in position -orto: the first substituent is a carboxylic acid and the second an ester group. The molecular geometry is planar because the phenyl ring and the carboxylic groups which have a sp2 hybridization. Its chemical structure can be written as below, in the common representations used for organic molecules.
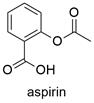
Occurrence: Aspirin can not be found in nature. It was synthesized by the first time in 1853 by the french chemist Charles Frédéric Gerhardt.
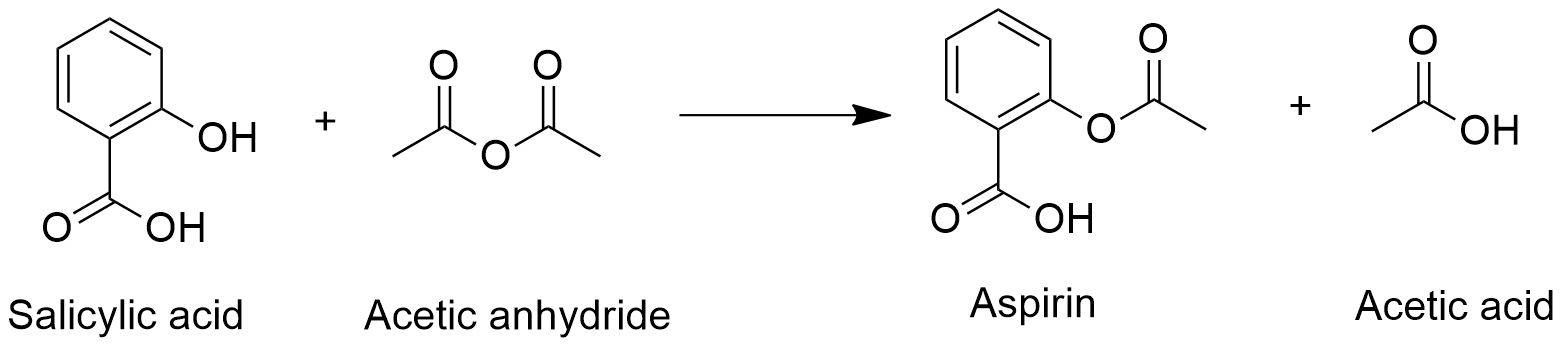
Preparation: Aspirin is synthesized through the esterification of salicylic acid by the acetic anhydride, thus the hydroxyl group in the salicylic acid is replaced by and ester group. The reaction can be catalyzed by sulfuric acid:
Physical properties: Acetylsalicylic acid is a colorless to white crystalline solid with a vinegar odor due its hydrolysis yielding salicylic and acetic acid. Aspirina has a bitter taste. Its density is 1.40 g mL-1. Its melting point is 135 ºC and in higher temperature it decomposes. It is soluble in water, ethanol, ethyl ether and chloroform.
Chemical properties: Aetylsalicyl acid is known by its capacity as anti-inflammatory drug. The mechanism of action is due the inhibition of enzyme cyclooxygenase, causing the suppression of Prostaglandin production (prostaglandins are molecules involved in inflammation process).
Uses: Acetylsalicylic acid is one of the most sold medicines around the world, since in 1897 Bayer laboratory gave the name of Aspirin and started its commercialization. It is used mainly as anti-inflammatory and anti-pyretic but in the last decades it has been a very popular in cardiovascular diseases treatment. Other uses includes the rheumatic fever and Kawasaki disease. Aspirin is also an intermediate and raw material in the production of other medicines or chemical compounds such as 4-hydroxycoumarin.
Health effects / safety hazards: Aspirin is stable at room temperature, however it should keep dry to avoid its hydrolysis. It can cause gastritis and ulceration when taken for a long time. It is incompatible with strong oxidizing agents and strong acids and bases.
|
Related Links: |
