Barium Oxide Formula
Barium oxide, also known as barium monoxide or baryte, is a chemical compound used as a component of electronic devices and catalysts.
Formula and structure: The barium oxide chemical formula is BaO. The molar mass is 153.33 g/mol. The molecule is formed by one barium cation Ba2+ and one oxide anion O2-. Both ions are bound by one ionic bond. The geometry of the molecule is octahedral. Its chemical structure can be written as below, in the common representations used for organic molecules.
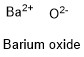
Occurrence: Barium oxide is not found in nature.
Preparation: Barium oxide can be prepared through two different methodologies:
a) The reaction of solid with oxygen gas at high temperatures:
2Ba + O2 → 2BaO
b) The decomposition of barium carbonate or barium nitrate at high temperatures:
BaCO3 → BaO + CO2
Physical properties: Barium oxide is a white solid. The density of this oxide is 5.72 g/mL(which is considered very heavy). Its melting point is 1923 °C and above this temperature, it boils. Barium oxide is soluble in water, where it reacts to form barium hydroxide. It is soluble in ethanol and acetone.
Chemical properties: Barium oxide can be used in the production of glasses due to it has a low refractive index, which means, the light travels slow through this material. When heated, the barium oxide can be used as a source of oxygen.
Uses: Barium oxide is used as a cathode for manufacturing cathode ray tubes, which were a TV-component in past. Barium is also used as catalyst for chemical reaction and can be used as a component in the production of some glasses.
Health effects / safety hazards: Barium oxide is extremely toxic for ingestion for human and environment. It should be avoid the contact with aquatic life. It can cause explosion when is in contact with other chemicals due to it Is a strong oxidizer.
|
Related Links: |
