Barium Phosphate Formula
Barium phosphate is an inorganic salt used in the chemical and optical industry as a component in the production of glasses and pulsed lasers.
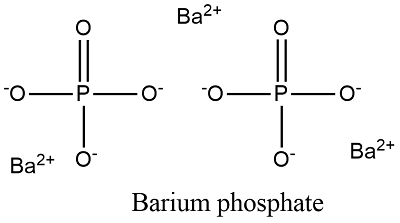
Formula and structure: The chemical structure of barium phosphate is Ba3(PO4)2 and its molecular weight is 295.27 g/mol. It is formed by 3 cations barium Ba2+ and 2 anions phosphate PO43-. It is also find in their hydrated forms Ba2(P4O12).5H2O, Ba3(P3O9)2· 6H2O. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Barium phosphate is found in minerals and rocks, for example, jagowerite and alforsite. In many of these minerals, barium phosphate is find forming heterogeneous mixture with ions as chloride.
Preparation: Barium phosphate is prepared by the reaction of the inorganic salts barium carbonate BaCO3 with the metaphosphoric acid.
BaCO3 + 2HPO3 → Ba(PO3)2 +CO2 +H2O
It can also be prepared with the salt barium chloride and sodium metaphosphate, where barium phosphate is precipitate and filtrated from the solution:
BaCl2(aq) + 2NaPO3(aq) → Ba(PO3)2 + 2NaCl
Physical properties: Barium phosphate is a colourless solid, commonly found as a powder. Its density is 3.63 g/mL and its melting point is 1560 ºC. Barium phosphate is insoluble in water, but it is soluble in acidic aqueous solutions.
Chemical properties: The chemical properties of barium phosphate can help to produce glasses with a high melting point and a high coefficient of thermal expansion. It is the reason that many industrial processes used for producing special glasses incorporate barium phosphate as a raw material.
Uses: Barium phosphate has several industrial applications, for example, it is used to produce a mixture of sodium and barium polyphosphate that are one of the component of hot glasses and other glasses with special properties. It is also used as a component of pulsed lasers and as a proton conducting material in fuel sensors.
Health effects/safety hazards: Barium phosphate is harmful if inhaled or swallowed. It can also cause skin and eyes irritation. It is not flammable.
|
Related Links: |
